Search for drugs:
Typing the drug name to query
INDACATEROL MALEATE
DIR Classification
Classification:Moderate-DIQT concern
Severity Score:3.0
Description in Drug Labeling: View Full Labeling: SPL in DailyMed | PDF
- WARNINGS AND PRECAUTIONS
- Cardiovascular Effects
- ARCAPTA NEOHALER, like other beta2-agonists, can produce a clinically significant cardiovascular effect in some patients as measured by increases in pulse rate, systolic or diastolic blood pressure, or symptoms. If such effects occur, ARCAPTA NEOHALER may need to be discontinued. In addition, beta-agonists have been reported to produce ECG changes, such as flattening of the T wave, prolongation of the QTc interval, and ST segment depression, although the clinical significance of these findings is unknown. Therefore, ARCAPTA NEOHALER, like other sympathomimetic amines, should be used with caution in patients with cardiovascular disorders, especially coronary insufficiency, cardiac arrhythmias, and hypertension.
- DRUG INTERACTIONS
- Monoamine Oxidase Inhibitors, Tricyclic Antidepressants, QTc Prolonging Drugs
- Indacaterol, as with other beta2-agonists, should be administered with extreme caution to patients being treated with monoamine oxidase inhibitors, tricyclic antidepressants, or other drugs known to prolong the QTc interval because the action of adrenergic agonists on the cardiovascular system may be potentiated by these agents. Drugs that are known to prolong the QTc interval may have an increased risk of ventricular arrhythmias.
- OVERDOSAGE
- Human Experience
- In COPD patients single doses of 40 times the 75 mcg dose were associated with moderate increases in pulse rate, systolic blood pressure and QTc interval.
- CLINICAL PHARMACOLOGY
- Pharmacodynamics
- Electrophysiology
- The effect of ARCAPTA NEOHALER on the QT interval was evaluated in a double-blind, placebo- and active (moxifloxacin)-controlled study following multiple doses of indacaterol 150 mcg, 300 mcg or 600 mcg once-daily for 2 weeks in 404 healthy volunteers. Fridericia's method for heart rate correction was employed to derive the corrected QT interval (QTcF). Maximum mean prolongation of QTcF intervals were <5 ms, and the upper limit of the 90% confidence interval was below 10 ms for all time-matched comparisons versus placebo. During these studies, there were no clinically meaningful QT-interval prolongations. There was no evidence of a clinically relevant concentration-delta QTc relationship in the range of doses evaluated.
Postmarketing Surveillance
Contingency Table:
Current Drug
Other Drugs
QT Prolongation
7
24085
Other ADRs
15128
38366459
Odds Ratio = 0.738
Drug Property Information
ATC Code(s):
- R03AC18 - indacaterol maleate
- R03AC - Selective beta-2-adrenoreceptor agonists
- R03A - "ADRENERGICS, INHALANTS"
- R03 - DRUGS FOR OBSTRUCTIVE AIRWAY DISEASES
- R - RESPIRATORY SYSTEM
Active Ingredient:INDACATEROL MALEATE
Active Ingredient UNII:2JEC1ITX7R
Drugbank ID:DB05039
PubChem Compound:6918554
CTD ID:C510790
PharmGKB:PA165958348
CAS Number:312753-06-3
Dosage Form(s):capsule
Route(s) Of Administrator:oral; respiratory (inhalation)
Daily Dose:
- 0.15 mg/day R03AC18
Chemical Structure: 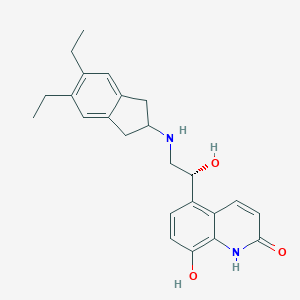

SMILE Code:
CCC1=C(CC)C=C2CC(CC2=C1)NC[C@H](O)C1=C2C=CC(=O)NC2=C(O)C=C1
CCC1=C(CC)C=C2CC(CC2=C1)NC[C@H](O)C1=C2C=CC(=O)NC2=C(O)C=C1
Reference
N/A
Disclaimer:
The content of this database of QT prolongation is intended for educational and scientific research purposes only. It is not intended as a substitute for professional medical advice, diagnosis or treatment.
Any mention of commercial products is for clarification and not intended as endorsement.