Search for drugs:
Typing the drug name to query
NELFINAVIR MESYLATE
DIR Classification
Classification:Moderate-DIQT concern
Severity Score:3.0
Description in Drug Labeling: View Full Labeling: SPL in DailyMed | PDF
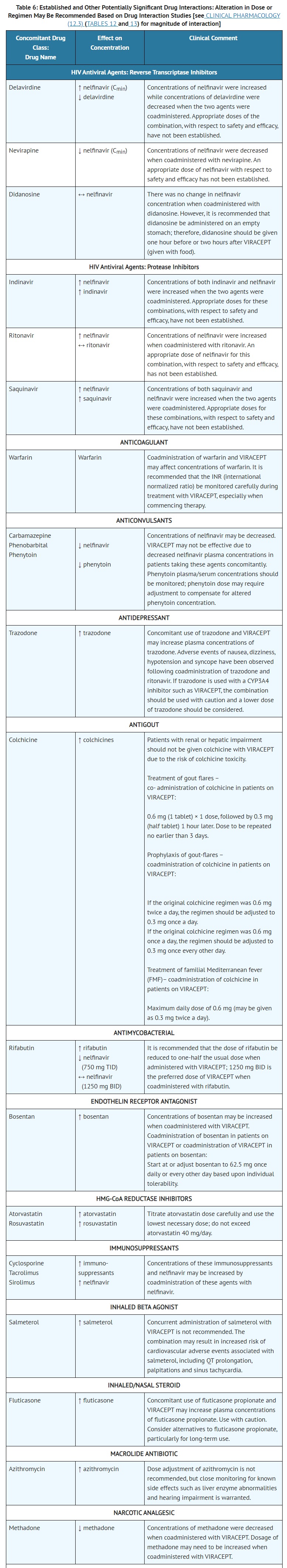
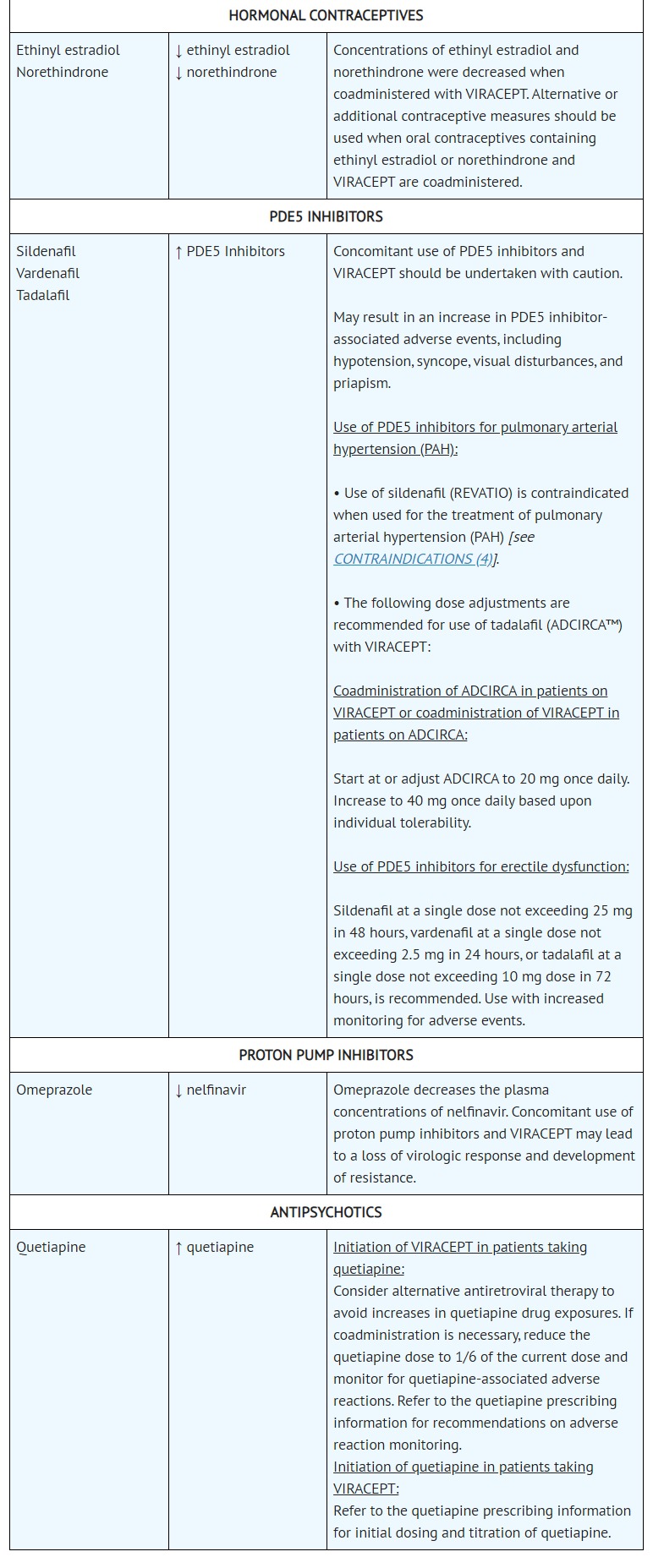
- DRUG INTERACTIONS
- ADVERSE REACTIONS
- Postmarketing Experience
- The following adverse reactions have been identified during post-approval use of VIRACEPT. Because these reactions are reported voluntarily from a population of uncertain size, it is not always possible to reliably estimate their frequency or establish a causal relationship to drug exposure.
- Cardiovascular System: QTc prolongation, torsades de pointes.
- CLINICAL PHARMACOLOGY
- Pharmacodynamics
- Effects on Electrocardiogram
- The effect of Viracept at the recommended dose of 1250 mg twice daily on the QTcF interval administered with a low fat meal (20% fat) was evaluated in a randomized, placebo and active (moxifloxacin 400 mg once daily) controlled, crossover study in 66 healthy subjects. The maximum mean time-matched (95% upper confidence bound) differences in QTcF interval from placebo after baseline-correction was below 10 milliseconds, the threshold of clinical concern. This finding was unchanged when a single supratherapeutic dose of Viracept 3125 mg was administered following a 3-day administration of Viracept 1250 mg twice daily. The exposure at 3125 mg was 1.4-fold that at 1250 mg. The dose of 3125 mg in this study did not achieve the anticipated exposures in patients taking a high fat meal (50% fat) or with concomitant administration of drugs that could increase nelfinavir exposure [see PHARMACOKINETICS (12.3)].
- No subject in any group had an increase in QTcF of ≥60 milliseconds from baseline. No subject experienced an interval exceeding the potentially clinically relevant threshold of 500 milliseconds.
Postmarketing Surveillance
Contingency Table:
Current Drug
Other Drugs
QT Prolongation
11
24081
Other ADRs
2208
38379379
Odds Ratio = 7.94
Drug Property Information
ATC Code(s):
- J05AE04 - nelfinavir mesylate
- J05AE - Protease inhibitors
- J05A - DIRECT ACTING ANTIVIRALS
- J05 - ANTIVIRALS FOR SYSTEMIC USE
- J - ANTIINFECTIVES FOR SYSTEMIC USE
Active Ingredient:NELFINAVIR MESYLATE
Active Ingredient UNII:98D603VP8V
Drugbank ID:DB00220
PubChem Compound:64143
CTD ID:D019888
PharmGKB:PA450606
CAS Number:159989-64-7
Dosage Form(s):tablet, film coated
Route(s) Of Administrator:oral
Daily Dose:
- 2250.0 mg/day J05AE04
Chemical Structure: 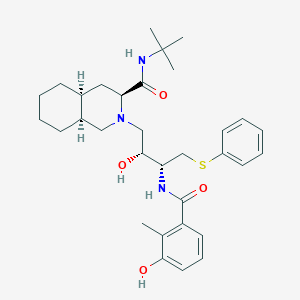

SMILE Code:
[H][C@@]12CCCC[C@]1([H])CN(C[C@@H](O)[C@H](CSC1=CC=CC=C1)NC(=O)C1=C(C)C(O)=CC=C1)[C@@H](C2)C(=O)NC(C)(C)C
[H][C@@]12CCCC[C@]1([H])CN(C[C@@H](O)[C@H](CSC1=CC=CC=C1)NC(=O)C1=C(C)C(O)=CC=C1)[C@@H](C2)C(=O)NC(C)(C)C
Reference
N/A
Disclaimer:
The content of this database of QT prolongation is intended for educational and scientific research purposes only. It is not intended as a substitute for professional medical advice, diagnosis or treatment.
Any mention of commercial products is for clarification and not intended as endorsement.