Search for drugs:
Typing the drug name to query
VORTIOXETINE
DIR Classification
Classification:Ambiguous
Severity Score:1.0
Description in Drug Labeling: View Full Labeling: SPL in DailyMed | PDF
- CLINICAL PHARMACOLOGY
- Pharmacodynamics
- Effect on Cardiac Repolarization
- The effect of vortioxetine 10 mg and 40 mg administered once daily on QTc interval was evaluated in a randomized, double-blind, placebo-, and active-controlled (moxifloxacin 400 mg), four-treatment-arm parallel study in 340 male subjects. In the study the upper bound of the one-sided 95% confidence interval for the QTc was below 10 ms, the threshold for regulatory concern. The oral dose of 40 mg is sufficient to assess the effect of metabolic inhibition.
Postmarketing Surveillance
Contingency Table:
Current Drug
Other Drugs
QT Prolongation
38
24054
Other ADRs
25307
38356280
Odds Ratio = 2.395
Drug Property Information
ATC Code(s):
- N06AX26 - vortioxetine
- N06AX2 -
- N06AX - Other antidepressants
- N06A - ANTIDEPRESSANTS
- N06 - PSYCHOANALEPTICS
- N - NERVOUS SYSTEM
Active Ingredient:VORTIOXETINE HYDROBROMIDE
Active Ingredient UNII:TKS641KOAY
Drugbank ID:DB09068
PubChem Compound:71768094
CTD ID:D000078784
PharmGKB:PA166122595
CAS Number:508233-74-7
Dosage Form(s):tablet, film coated
Route(s) Of Administrator:oral
Daily Dose:
- 10.0 mg/day N06AX26
Chemical Structure: 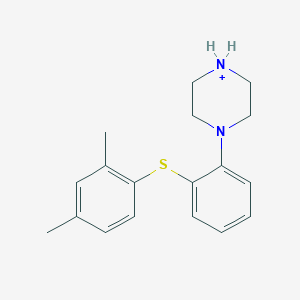

SMILE Code:
CC1=CC=C(SC2=CC=CC=C2N2CCNCC2)C(C)=C1
CC1=CC=C(SC2=CC=CC=C2N2CCNCC2)C(C)=C1
Reference
1: QT prolongation and vortioxetine: a post-marketing study and comparison with other serotonin reuptake inhibitors.
[Bordet Constance,Rousseau Vanessa,Montastruc François,Montastruc Jean-Louis]Psychopharmacology (Berl),2020 Apr;237(4):1245-1247. PMID: 31965253
2: Adverse Effects of Pharmacologic Treatments of Major Depression in Older Adults.
[Sobieraj Diana M,Martinez Brandon K,Hernandez Adrian V,Coleman Craig I,Ross Joseph S,Berg Karina M,Steffens David C,Baker William L]J Am Geriatr Soc,2019 Aug;67(8):1571-1581. PMID: 31140587
Disclaimer:
The content of this database of QT prolongation is intended for educational and scientific research purposes only. It is not intended as a substitute for professional medical advice, diagnosis or treatment.
Any mention of commercial products is for clarification and not intended as endorsement.