Search for drugs:
Typing the drug name to query
METHYLPHENIDATE HYDROCHLORIDE
DIR Classification
Classification:Ambiguous
Severity Score:1.0
Description in Drug Labeling: View Full Labeling: SPL in DailyMed | PDF
- CLINICAL PHARMACOLOGY
- Pharmacodynamics
- Cardiac Electrophysiology
- A formal QT study has not been conducted in patients taking methylphenidate hydrochloride tablets.
- The effect of dexmethylphenidate, the pharmacologically active d-enantiomer of methylphenidate hydrochloride tablets, on the QT interval was evaluated in a double-blind, placebo- and open-label active (moxifloxacin)-controlled study following single doses of dexmethlyphenidate XR 40 mg (maximum recommended adult total daily dosage) in 75 healthy volunteers. Electrocardiograms (ECGs) were collected up to 12 hours postdose. Frederica’s method for heart rate correction was employed to derive the corrected QT interval (QTcF). The maximum mean prolongation of QTcF intervals was less than 5 ms, and the upper limit of the 90% confidence interval (CI) was below 10 ms for all time-matched comparisons versus placebo. This was below the threshold of clinical concern and there was no evident exposure response relationship.
Postmarketing Surveillance
Contingency Table:
Current Drug
Other Drugs
QT Prolongation
91
24001
Other ADRs
97295
38284292
Odds Ratio = 1.492
Drug Property Information
ATC Code(s):
- N06BA04 - methylphenidate hydrochloride
- N06BA - Centrally acting sympathomimetics
- N06B - "PSYCHOSTIMULANTS, AGENTS USED FOR ADHD AND NOOTROPICS"
- N06 - PSYCHOANALEPTICS
- N - NERVOUS SYSTEM
Active Ingredient:METHYLPHENIDATE HYDROCHLORIDE
Active Ingredient UNII:4B3SC438HI
Drugbank ID:DB00422
PubChem Compound:4158
CTD ID:D008774
PharmGKB:PA450464
CAS Number:113-45-1
Dosage Form(s):tablet
Route(s) Of Administrator:oral
Daily Dose:
- 30.0 mg/day N06BA04
Chemical Structure: 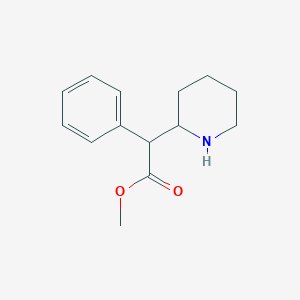

SMILE Code:
COC(=O)C(C1CCCCN1)C1=CC=CC=C1
COC(=O)C(C1CCCCN1)C1=CC=CC=C1
Reference
N/A
Disclaimer:
The content of this database of QT prolongation is intended for educational and scientific research purposes only. It is not intended as a substitute for professional medical advice, diagnosis or treatment.
Any mention of commercial products is for clarification and not intended as endorsement.