Search for drugs:
Typing the drug name to query
GRANISETRON HYDROCHLORIDE
DIR Classification
Classification:Moderate-DIQT concern
Severity Score:3.0
Description in Drug Labeling: View Full Labeling: SPL in DailyMed | PDF
- PRECAUTIONS
- Granisetron is not a drug that stimulates gastric or intestinal peristalsis. It should not be used instead of nasogastric suction. The use of granisetron in patients following abdominal surgery or in patients with chemotherapy-induced nausea and vomiting may mask a progressive ileus and/or gastric distention.
- An adequate QT assessment has not been conducted, but QT prolongation has been reported with granisetron hydrochloride. Therefore, granisetron hydrochloride should be used with caution in patients with pre-existing arrhythmias or cardiac conduction disorders, as this might lead to clinical consequences. Patients with cardiac disease, on cardio-toxic chemotherapy, with concomitant electrolyte abnormalities and/or on concomitant medications that prolong the QT interval are particularly at risk.
- [Drug Interactions]
- QT prolongation has been reported with granisetron hydrochloride. Use of granisetron hydrochloride in patients concurrently treated with drugs known to prolong the QT interval and/or are arrhythmogenic may result in clinical consequences.
- ADVERSE REACTIONS
- QT prolongation has been reported with granisetron hydrochloride (see PRECAUTIONS and DRUG INTERACTIONS).
- [Postmarketing Experience]
- QT prolongation has been reported with granisetron hydrochloride (see PRECAUTIONS and DRUG INTERACTIONS).
Postmarketing Surveillance
Contingency Table:
Current Drug
Other Drugs
QT Prolongation
8
24084
Other ADRs
4572
38377015
Odds Ratio = 2.789
Drug Property Information
ATC Code(s):
- A04AA02 - granisetron hydrochloride
- A04AA - Serotonin (5HT3) antagonists
- A04A - ANTIEMETICS AND ANTINAUSEANTS
- A04 - ANTIEMETICS AND ANTINAUSEANTS
- A - ALIMENTARY TRACT AND METABOLISM
Active Ingredient:GRANISETRON HYDROCHLORIDE
Active Ingredient UNII:318F6L70J8
Drugbank ID:DB00889
PubChem Compound:5284566
CTD ID:D017829
PharmGKB:PA449809
CAS Number:109889-09-0
Dosage Form(s):tablet
Route(s) Of Administrator:oral
Daily Dose:
- 2.0 mg/day A04AA02
Chemical Structure: 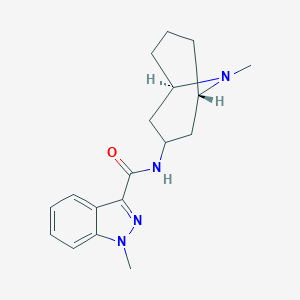

SMILE Code:
CN1N=C(C(=O)N[C@@H]2C[C@@H]3CCC[C@H](C2)N3C)C2=C1C=CC=C2
CN1N=C(C(=O)N[C@@H]2C[C@@H]3CCC[C@H](C2)N3C)C2=C1C=CC=C2
Reference
N/A
Disclaimer:
The content of this database of QT prolongation is intended for educational and scientific research purposes only. It is not intended as a substitute for professional medical advice, diagnosis or treatment.
Any mention of commercial products is for clarification and not intended as endorsement.