Search for drugs:
Typing the drug name to query
LEVOCETIRIZINE DIHYDROCHLORIDE
DIR Classification
Classification:Ambiguous
Severity Score:1.0
Description in Drug Labeling: View Full Labeling: SPL in DailyMed | PDF
- CLINICAL PHARMACOLOGY
- Pharmacodynamics
- A QT/QTc study using a single dose of 30 mg of levocetirizine did not demonstrate an effect on the QTc interval. While a single dose of levocetirizine had no effect, the effects of levocetirizine may not be at steady state following single dose. The effect of levocetirizine on the QTc interval following multiple dose administration is unknown. Levocetirizine is not expected to have QT/QTc effects because of the results of QTc studies with cetirizine and the long postmarketing history of cetirizine without reports of QT prolongation.
Postmarketing Surveillance
Contingency Table:
Current Drug
Other Drugs
QT Prolongation
14
24078
Other ADRs
11647
38369940
Odds Ratio = 1.916
Drug Property Information
ATC Code(s):
- R06AE09 - levocetirizine dihydrochloride
- R06AE0 -
- R06AE - Piperazine derivatives
- R06A - ANTIHISTAMINES FOR SYSTEMIC USE
- R06 - ANTIHISTAMINES FOR SYSTEMIC USE
- R - RESPIRATORY SYSTEM
Active Ingredient:Levocetirizine dihydrochloride
Active Ingredient UNII:SOD6A38AGA
Drugbank ID:DB06282
PubChem Compound:1549000
CTD ID:C472067
CAS Number:130018-77-8
Dosage Form(s):solution
Route(s) Of Administrator:oral
Daily Dose:
- 5.0 mg/day R06AE09
Chemical Structure: 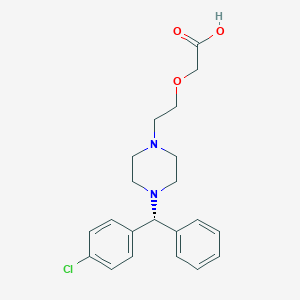

SMILE Code:
OC(=O)COCCN1CCN(CC1)[C@H](C1=CC=CC=C1)C1=CC=C(Cl)C=C1
OC(=O)COCCN1CCN(CC1)[C@H](C1=CC=CC=C1)C1=CC=C(Cl)C=C1
Reference
N/A
Disclaimer:
The content of this database of QT prolongation is intended for educational and scientific research purposes only. It is not intended as a substitute for professional medical advice, diagnosis or treatment.
Any mention of commercial products is for clarification and not intended as endorsement.