Search for drugs:
Typing the drug name to query
FOSPHENYTOIN SODIUM
DIR Classification
Classification:Most-DIQT concern
Severity Score:4.0
Description in Drug Labeling: View Full Labeling: SPL in DailyMed | PDF
- WARNINGS AND PRECAUTIONS
- Cardiovascular Risk Associated with Rapid Infusion
- Rapid intravenous administration of CEREBYX increases the risk of adverse cardiovascular reactions, including severe hypotension and cardiac arrhythmias. Cardiac arrhythmias have included bradycardia, heart block, QT interval prolongation, ventricular tachycardia, and ventricular fibrillation which have resulted in asystole, cardiac arrest, and death. Severe complications are most commonly encountered in critically ill patients, elderly patients, and patients with hypotension and severe myocardial insufficiency. However, cardiac events have also been reported in adults and children without underlying cardiac disease or comorbidities and at recommended doses and infusion rates.
- ADVERSE REACTIONS
- Clinical Trials Experience
- Cardiovascular: Frequent: hypertension; Infrequent: cardiac arrest, migraine, syncope, cerebral hemorrhage, palpitation, sinus bradycardia, atrial flutter, bundle branch block, cardiomegaly, cerebral infarct, postural hypotension, pulmonary embolus, QT interval prolongation, thrombophlebitis, ventricular extrasystoles, congestive heart failure.
Postmarketing Surveillance
Contingency Table:
Current Drug
Other Drugs
QT Prolongation
2
24090
Other ADRs
2144
38379443
Odds Ratio = 1.487
Drug Property Information
ATC Code(s):
- N03AB05 - fosphenytoin sodium
- N03AB - Hydantoin derivatives
- N03A - ANTIEPILEPTICS
- N03 - ANTIEPILEPTICS
- N - NERVOUS SYSTEM
Active Ingredient:FOSPHENYTOIN SODIUM
Active Ingredient UNII:7VLR55452Z
Drugbank ID:DB00252
PubChem Compound:1775
CTD ID:D010672
PharmGKB:PA450947
CAS Number:57-41-0
Dosage Form(s):injection, solution
Route(s) Of Administrator:intramuscular; intravenous
Daily Dose:
- 450.0 mg/day N03AB05
Chemical Structure: 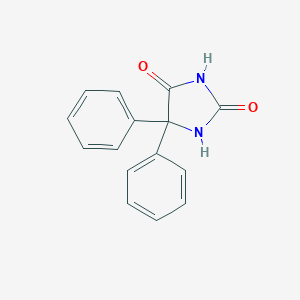

SMILE Code:
O=C1NC(=O)C(N1)(C1=CC=CC=C1)C1=CC=CC=C1
O=C1NC(=O)C(N1)(C1=CC=CC=C1)C1=CC=CC=C1
Reference
N/A
Disclaimer:
The content of this database of QT prolongation is intended for educational and scientific research purposes only. It is not intended as a substitute for professional medical advice, diagnosis or treatment.
Any mention of commercial products is for clarification and not intended as endorsement.