Search for drugs:
Typing the drug name to query
LUMATEPERONE
DIR Classification
Classification:Ambiguous
Severity Score:1.0
Description in Drug Labeling: View Full Labeling: SPL in DailyMed | PDF
- CLINICAL PHARMACOLOGY
- Pharmacodynamics
- Cardiac Electrophysiology
- QTcF interval was evaluated in a randomized, placebo- and active- (moxifloxacin 400 mg) controlled, four-arm crossover study utilizing concentration-QTc effect modeling in 33 patients with schizophrenia. The placebo-corrected change from baseline QTcF (90% two-sided upper confidence interval) values of 4.9 (8.9) and 15.8 (19.8) ms for the 42 mg and the supratherapeutic dose of 126 mg (three times the recommended daily dosage) CAPLYTA, respectively, administered orally once daily for 5 days.
Postmarketing Surveillance
Contingency Table:
Current Drug
Other Drugs
QT Prolongation
0
24092
Other ADRs
0
38381587
Odds Ratio = N/A
Drug Property Information
ATC Code(s):
Active Ingredient:LUMATEPERONE
Active Ingredient UNII:70BSQ12069
Drugbank ID:DB06077
PubChem Compound:21302490
CTD ID:C000705749
CAS Number:313368-91-1
Dosage Form(s):capsule
Route(s) Of Administrator:oral
Daily Dose:
Chemical Structure: 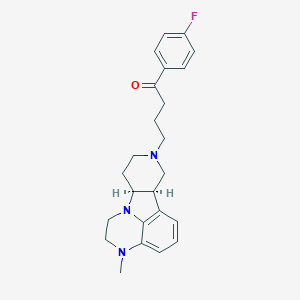

SMILE Code:
[H][C@]12CCN(CCCC(=O)C3=CC=C(F)C=C3)C[C@@]1([H])C1=CC=CC3=C1N2CCN3C
[H][C@]12CCN(CCCC(=O)C3=CC=C(F)C=C3)C[C@@]1([H])C1=CC=CC3=C1N2CCN3C
Reference
N/A
Disclaimer:
The content of this database of QT prolongation is intended for educational and scientific research purposes only. It is not intended as a substitute for professional medical advice, diagnosis or treatment.
Any mention of commercial products is for clarification and not intended as endorsement.