Search for drugs:
Typing the drug name to query
TIZANIDINE
DIR Classification
Classification:Ambiguous
Severity Score:1.0
Description in Drug Labeling: View Full Labeling: SPL in DailyMed | PDF
- PRECAUTIONS
- CARDIOVASCULAR
- Prolongation of the QT interval and bradycardia were noted in chronic toxicity studies in dogs at doses equal to the maximum human dose on a mg/m 2 basis. ECG evaluation was not performed in the controlled clinical studies. Reduction in pulse rate has been noted in association with decreases in blood pressure in the single dose controlled study (see WARNINGS).
Postmarketing Surveillance
Contingency Table:
Current Drug
Other Drugs
QT Prolongation
16
24076
Other ADRs
6471
38375116
Odds Ratio = 3.942
Drug Property Information
ATC Code(s):
- M03BX02 - tizanidine
- M03BX - Other centrally acting agents
- M03B - "MUSCLE RELAXANTS, CENTRALLY ACTING AGENTS"
- M03 - MUSCLE RELAXANTS
- M - MUSCULO-SKELETAL SYSTEM
Active Ingredient:TIZANIDINE HYDROCHLORIDE
Active Ingredient UNII:B53E3NMY5C
Drugbank ID:DB00697
PubChem Compound:5487
CTD ID: C023754
PharmGKB:PA451701
CAS Number:51322-75-9
Dosage Form(s):tablet
Route(s) Of Administrator:oral
Daily Dose:
- 12.0 mg/day M03BX02
Chemical Structure: 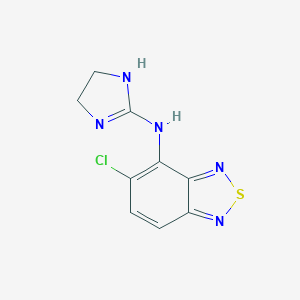

SMILE Code:
ClC1=C(NC2=NCCN2)C2=NSN=C2C=C1
ClC1=C(NC2=NCCN2)C2=NSN=C2C=C1
Reference
1: Potassium wasting nephropathy in the setting of tizanidine overdose: a case report.
[Brucculeri Michael J,Garcia Juan]J Med Case Rep,2021 May 1;15(1):250. PMID: 33931107
2: Drug safety of macrolide and quinolone antibiotics in a tertiary care hospital: administration of interacting co-medication and QT prolongation.
[Niedrig David,Maechler Sarah,Hoppe Liesa,Corti Natascia,Kovari Helen,Russmann Stefan]Eur J Clin Pharmacol,2016 Jul;72(7):859-67. PMID: 27023463
3: Drug-induced QT prolongation and sudden death.
[Del Rosario Marc E,Weachter Richard,Flaker Greg C]Mo Med,Jan-Feb 2010;107(1):53-8. PMID: 20222297
Disclaimer:
The content of this database of QT prolongation is intended for educational and scientific research purposes only. It is not intended as a substitute for professional medical advice, diagnosis or treatment.
Any mention of commercial products is for clarification and not intended as endorsement.