Search for drugs:
Typing the drug name to query
POTASSIUM CITRATE
DIR Classification
Classification:Moderate-DIQT concern
Severity Score:2.0
Description in Drug Labeling: View Full Labeling: SPL in DailyMed | PDF
- OVERDOSAGE
- Treatment of Overdosage: The administration of potassium salts to persons without predisposing conditions for hyperkalemia rarely causes serious hyperkalemia at recommended dosages. It is important to recognize that hyperkalemia is usually asymptomatic and may be manifested only by an increased serum potassium concentration and characteristic electrocardiographic changes (peaking of T-wave, loss of P-wave, depression of S-T segment and prolongation of the QT interval). Late manifestations include muscle paralysis and cardiovascular collapse from cardiac arrest.
Postmarketing Surveillance
Contingency Table:
Current Drug
Other Drugs
QT Prolongation
0
24092
Other ADRs
493
38381094
Odds Ratio = 0.0
Drug Property Information
ATC Code(s):
- A12BA02 - potassium citrate
- A12BA - Potassium
- A12B - POTASSIUM
- A12 - MINERAL SUPPLEMENTS
- A - ALIMENTARY TRACT AND METABOLISM
Active Ingredient:POTASSIUM CITRATE
Active Ingredient UNII:EE90ONI6FF
Drugbank ID:DB01345
PubChem Compound:5462222
CTD ID:D019357
PharmGKB:PA451047
CAS Number:24203-36-9
Dosage Form(s):tablet, extended release
Route(s) Of Administrator:oral
Daily Dose:
- 4.0 mg/day A12BA02
Chemical Structure: 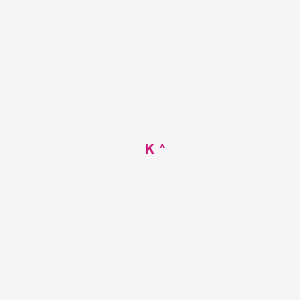

SMILE Code:
[K+]
[K+]
Reference
N/A
Disclaimer:
The content of this database of QT prolongation is intended for educational and scientific research purposes only. It is not intended as a substitute for professional medical advice, diagnosis or treatment.
Any mention of commercial products is for clarification and not intended as endorsement.