Search for drugs:
Typing the drug name to query
NERATINIB
DIR Classification
Classification:Ambiguous
Severity Score:1.0
Description in Drug Labeling: View Full Labeling: SPL in DailyMed | PDF
- CLINICAL PHARMACOLOGY
- Pharmacodynamics
- Cardiac Electrophysiology
- The effect of NERLYNX on the QTc interval was evaluated in a randomized, placebo, and positive-controlled, double-blind, single-dose, crossover study in 60 healthy subjects. At 140% the therapeutic exposures of NERLYNX, there was no clinically relevant effect on the QTc interval.
Postmarketing Surveillance
Contingency Table:
Current Drug
Other Drugs
QT Prolongation
1
24091
Other ADRs
6242
38375345
Odds Ratio = 0.256
Drug Property Information
ATC Code(s):
- L01EH02 - neratinib
- L01EH -
- L01E -
- L01 - ANTINEOPLASTIC AGENTS
- L - ANTINEOPLASTIC AND IMMUNOMODULATING AGENTS
Active Ingredient:NERATINIB MALEATE
Active Ingredient UNII:9RM7XY23ZS
Drugbank ID:DB11828
PubChem Compound:9915743
CTD ID: C487932
CAS Number:698387-09-6
Dosage Form(s):tablet
Route(s) Of Administrator:oral
Daily Dose:
Chemical Structure: 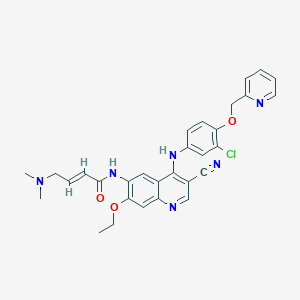

SMILE Code:
CCOC1=C(NC(=O)\C=C\CN(C)C)C=C2C(NC3=CC=C(OCC4=CC=CC=N4)C(Cl)=C3)=C(C=NC2=C1)C#N
CCOC1=C(NC(=O)\C=C\CN(C)C)C=C2C(NC3=CC=C(OCC4=CC=CC=N4)C(Cl)=C3)=C(C=NC2=C1)C#N
Reference
1: Neratinib after trastuzumab-based adjuvant therapy in patients with HER2-positive breast cancer (ExteNET): a multicentre, randomised, double-blind, placebo-controlled, phase 3 trial.
[Chan Arlene,Delaloge Suzette,Holmes Frankie A,Moy Beverly,Iwata Hiroji,Harvey Vernon J,Robert Nicholas J,Silovski Tajana,Gokmen Erhan,von Minckwitz Gunter,Ejlertsen Bent,Chia Stephen K L,Mansi Janine,Barrios Carlos H,Gnant Michael,Buyse Marc,Gore Ira,Smith John,Harker Graydon,Masuda Norikazu,Petrakova Katarina,Zotano Angel Guerrero,Iannotti Nicholas,Rodriguez Gladys,Tassone Pierfrancesco,Wong Alvin,Bryce Richard,Ye Yining,Yao Bin,Martin Miguel,ExteNET Study Group]Lancet Oncol,2016 Mar;17(3):367-377. PMID: 26874901
Disclaimer:
The content of this database of QT prolongation is intended for educational and scientific research purposes only. It is not intended as a substitute for professional medical advice, diagnosis or treatment.
Any mention of commercial products is for clarification and not intended as endorsement.