Search for drugs:
Typing the drug name to query
VORINOSTAT
DIR Classification
Classification:Ambiguous
Severity Score:1.0
Description in Drug Labeling: View Full Labeling: SPL in DailyMed | PDF
- CLINICAL PHARMACOLOGY
- Pharmacodynamics
- Cardiac electrophysiology
- A randomized, partially-blind, placebo-controlled, 2-period crossover study was performed to assess the effects of a single 800-mg dose of vorinostat on the QTc interval in 24 patients with advanced cancer. This study was conducted to assess the impact of vorinostat on ventricular repolarization. The upper bound of the 90% confidence interval of the placebo-adjusted mean QTc interval change-from-baseline was less than 10 msec at every time point through 24 hours. Based on these study results, administration of a single supratherapeutic 800-mg dose of vorinostat does not appear to prolong the QTc interval in patients with advanced cancer; however the study did not include a positive control to demonstrate assay sensitivity. In the fasted state, oral administration of a single 800-mg dose of vorinostat resulted in a mean AUC and Cmax and median Tmax of 8.6±5.7 µM∙hr and 1.7±0.67 µM and 2.1 (0.5-6) hours, respectively.
- In clinical studies in patients with CTCL, three of 86 CTCL patients exposed to 400 mg once daily had Grade 1 (>450-470 msec) or 2 (>470-500 msec or increase of >60 msec above baseline) clinical adverse reactions of QTc prolongation. In a retrospective analysis of three Phase 1 and two Phase 2 studies, 116 patients had a baseline and at least one follow-up ECG. Four patients had Grade 2 (>470-500 msec or increase of >60 msec above baseline) and 1 patient had Grade 3 (>500 msec) QTc prolongation. In 49 non-CTCL patients from 3 clinical trials who had complete evaluation of QT interval, 2 had QTc measurements of >500 msec and 1 had a QTc prolongation of >60 msec.
Postmarketing Surveillance
Contingency Table:
Current Drug
Other Drugs
QT Prolongation
13
24079
Other ADRs
4004
38377583
Odds Ratio = 5.175
Drug Property Information
ATC Code(s):
- L01XH01 - vorinostat
- L01XH -
- L01X - OTHER ANTINEOPLASTIC AGENTS
- L01 - ANTINEOPLASTIC AGENTS
- L - ANTINEOPLASTIC AND IMMUNOMODULATING AGENTS
Active Ingredient:VORINOSTAT
Active Ingredient UNII:58IFB293JI
Drugbank ID:DB02546
PubChem Compound:5311
CTD ID: D000077337
PharmGKB:PA164748224
CAS Number:149647-78-9
Dosage Form(s):capsule
Route(s) Of Administrator:oral
Daily Dose:
Chemical Structure: 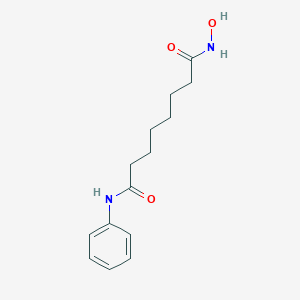

SMILE Code:
ONC(=O)CCCCCCC(=O)NC1=CC=CC=C1
ONC(=O)CCCCCCC(=O)NC1=CC=CC=C1
Reference
1: A phase 1 clinical trial of vorinostat in combination with decitabine in patients with acute myeloid leukaemia or myelodysplastic syndrome.
[Kirschbaum Mark,Gojo Ivana,Goldberg Stuart L,Bredeson Christopher,Kujawski Lisa A,Yang Allen,Marks Peter,Frankel Paul,Sun Xing,Tosolini Alessandra,Eid Joseph E,Lubiniecki Gregory M,Issa Jean-Pierre]Br J Haematol,2014 Oct;167(2):185-93. PMID: 25040094
2: A phase I trial of vorinostat and alvocidib in patients with relapsed, refractory, or poor prognosis acute leukemia, or refractory anemia with excess blasts-2.
[Holkova Beata,Supko Jeffrey G,Ames Matthew M,Reid Joel M,Shapiro Geoffrey I,Perkins Edward Brent,Ramakrishnan Viswanathan,Tombes Mary Beth,Honeycutt Connie,McGovern Renee M,Kmieciak Maciej,Shrader Ellen,Wellons Martha D,Sankala Heidi,Doyle Austin,Wright John,Roberts John D,Grant Steven]Clin Cancer Res,2013 Apr 1;19(7):1873-83. PMID: 23515411
3: QT interval prolongation and torsades de pointes in a patient undergoing treatment with vorinostat: a case report and review of the literature.
[Lynch Donald R,Washam Jeffrey B,Newby L Kristin]Cardiol J,2012;19(4):434-8. PMID: 22825908
Disclaimer:
The content of this database of QT prolongation is intended for educational and scientific research purposes only. It is not intended as a substitute for professional medical advice, diagnosis or treatment.
Any mention of commercial products is for clarification and not intended as endorsement.