Search for drugs:
Typing the drug name to query
ETELCALCETIDE
DIR Classification
Classification:Most-DIQT concern
Severity Score:4.0
Description in Drug Labeling: View Full Labeling: SPL in DailyMed | PDF
- WARNINGS AND PRECAUTIONS
- Hypocalcemia
- PARSABIV lowers serum calcium [see Adverse Reactions (6.1)] and can lead to hypocalcemia, sometimes severe. Significant lowering of serum calcium can cause paresthesias, myalgias, muscle spasms, seizures, QT interval prolongation, and ventricular arrhythmia.
- QT Interval Prolongation and Ventricular Arrhythmia
- In the combined placebo-controlled studies, more patients treated with PARSABIV experienced a maximum increase from baseline of greater than 60 msec in the QTcF interval (0% placebo versus 1.2% PARSABIV). In these studies, the incidence of a maximum post-baseline predialysis QTcF > 500 msec in the placebo and PARSABIV groups was 1.9% and 4.8%, respectively [see Adverse Reactions (6.1)]. Patients with congenital long QT syndrome, history of QT interval prolongation, family history of long QT syndrome or sudden cardiac death, and other conditions that predispose to QT interval prolongation and ventricular arrhythmia may be at increased risk for QT interval prolongation and ventricular arrhythmias if they develop hypocalcemia due to PARSABIV. Closely monitor corrected serum calcium and QT interval in patients at risk receiving PARSABIV.
- ADVERSE REACTIONS
- Clinical Trials Experience
- QTc Interval Prolongation Secondary to Hypocalcemia
- In the combined placebo-controlled studies, more patients treated with PARSABIV experienced a maximum increase from baseline of greater than 60 msec in the QTcF interval (0% placebo versus 1.2% PARSABIV). The patient incidence of maximum post-baseline predialysis QTcF > 500 msec in the placebo and PARSABIV groups was 1.9% and 4.8%, respectively.
Postmarketing Surveillance
Contingency Table:
Current Drug
Other Drugs
QT Prolongation
10
24082
Other ADRs
3813
38377774
Odds Ratio = 4.18
Drug Property Information
ATC Code(s):
- H05BX04 - etelcalcetide
- H05BX0 -
- H05BX - Other anti-parathyroid agents
- H05B - ANTI-PARATHYROID AGENTS
- H05 - CALCIUM HOMEOSTASIS
- H - "SYSTEMIC HORMONAL PREPARATIONS, EXCL. "
Active Ingredient:ETELCALCETIDE HYDROCHLORIDE
Active Ingredient UNII:72PT5993DU
Drugbank ID:DB12865
PubChem Compound:71511839
CTD ID: C583569
CAS Number:1262780-97-1
Dosage Form(s):injection, solution
Route(s) Of Administrator:intravenous
Daily Dose:
- 2.1 mg/day H05BX04
Chemical Structure: 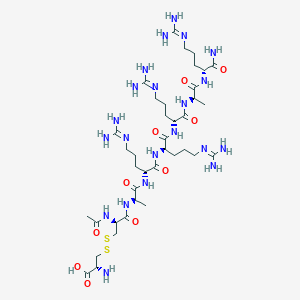

SMILE Code:
C[C@@H](NC(=O)[C@@H](CCCNC(N)=N)NC(=O)[C@@H](CCCNC(N)=N)NC(=O)[C@@H](CCCNC(N)=N)NC(=O)[C@@H](C)NC(=O)[C@@H](CSSC[C@H](N)C(O)=O)NC(C)=O)C(=O)N[C@H](CCCNC(N)=N)C(N)=O
C[C@@H](NC(=O)[C@@H](CCCNC(N)=N)NC(=O)[C@@H](CCCNC(N)=N)NC(=O)[C@@H](CCCNC(N)=N)NC(=O)[C@@H](C)NC(=O)[C@@H](CSSC[C@H](N)C(O)=O)NC(C)=O)C(=O)N[C@H](CCCNC(N)=N)C(N)=O
Reference
1: Nonclinical Safety Profile of Etelcalcetide, a Novel Peptide Calcimimetic for the Treatment of Secondary Hyperparathyroidism.
[Fielden Mark R,Dean Charles,Black Kurt,Sawant Satin G,Subramanian Raju,Tomlinson James E,Walter Sarah,Zimmermann Cameron,Griggs Mark W,McKeon Marie E,Lewis Elise M,Beevers Carol,Pyrah Ian]Int J Toxicol,2016 May;35(3):294-308. PMID: 26941242
Disclaimer:
The content of this database of QT prolongation is intended for educational and scientific research purposes only. It is not intended as a substitute for professional medical advice, diagnosis or treatment.
Any mention of commercial products is for clarification and not intended as endorsement.