Search for drugs:
Typing the drug name to query
LINAGLIPTIN
DIR Classification
Classification:Ambiguous
Severity Score:1.0
Description in Drug Labeling: View Full Labeling: SPL in DailyMed | PDF
- CLINICAL PHARMACOLOGY
- Pharmacodynamics
- Cardiac Electrophysiology
- In a randomized, placebo-controlled, active-comparator, 4-way crossover study, 36 healthy subjects were administered a single oral dose of linagliptin 5 mg, linagliptin 100 mg (20 times the recommended dose), moxifloxacin, and placebo. No increase in QTc was observed with either the recommended dose of 5 mg or the 100-mg dose. At the 100-mg dose, peak linagliptin plasma concentrations were approximately 38-fold higher than the peak concentrations following a 5-mg dose.
Postmarketing Surveillance
Contingency Table:
Current Drug
Other Drugs
QT Prolongation
4
24088
Other ADRs
14171
38367416
Odds Ratio = 0.45
Drug Property Information
ATC Code(s):
- A10BH05 - linagliptin
- A10BH0 -
- A10BH - Dipeptidyl peptidase 4 (DPP-4) inhibitors
- A10B - "BLOOD GLUCOSE LOWERING DRUGS, EXCL. INSULINS"
- A10 - DRUGS USED IN DIABETES
- A - ALIMENTARY TRACT AND METABOLISM
- A10BD19 - linagliptin
- A10BD - Combinations of oral blood glucose lowering drugs
- A10B - "BLOOD GLUCOSE LOWERING DRUGS, EXCL. INSULINS"
- A10 - DRUGS USED IN DIABETES
- A - ALIMENTARY TRACT AND METABOLISM
- A10BD11 - linagliptin
- A10BD - Combinations of oral blood glucose lowering drugs
- A10B - "BLOOD GLUCOSE LOWERING DRUGS, EXCL. INSULINS"
- A10 - DRUGS USED IN DIABETES
- A - ALIMENTARY TRACT AND METABOLISM
Active Ingredient:LINAGLIPTIN
Active Ingredient UNII:3X29ZEJ4R2
Drugbank ID:DB08882
PubChem Compound:10096344
CTD ID: D000069476
CAS Number:668270-12-0
Dosage Form(s):tablet, film coated
Route(s) Of Administrator:oral
Daily Dose:
- 5.0 mg/day A10BH05
Chemical Structure: 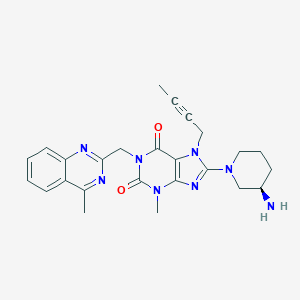

SMILE Code:
CC#CCN1C(=NC2=C1C(=O)N(CC1=NC3=C(C=CC=C3)C(C)=N1)C(=O)N2C)N1CCC[C@@H](N)C1
CC#CCN1C(=NC2=C1C(=O)N(CC1=NC3=C(C=CC=C3)C(C)=N1)C(=O)N2C)N1CCC[C@@H](N)C1
Reference
1: Pharmacokinetic interaction between linagliptin and tadalafil in healthy Egyptian males using a novel LC-MS method.
[Mourad Sara S,El-Kimary Eman I,Barary Magda A,Hamdy Dalia A]Bioanalysis,2019 Jul;11(14):1321-1336. PMID: 31368774
2: Evaluation system for arrhythmogenic potential of drugs using human-induced pluripotent stem cell-derived cardiomyocytes and gene expression analysis.
[Higa Arisa,Hoshi Hirotaka,Yanagisawa Yuka,Ito Emi,Morisawa Gaku,Imai Jun-Ichi,Watanabe Shinya,Takagi Motoki]J Toxicol Sci,2017;42(6):755-761. PMID: 29142174
Disclaimer:
The content of this database of QT prolongation is intended for educational and scientific research purposes only. It is not intended as a substitute for professional medical advice, diagnosis or treatment.
Any mention of commercial products is for clarification and not intended as endorsement.