Search for drugs:
Typing the drug name to query
METHYLNALTREXONE BROMIDE
DIR Classification
Classification:Ambiguous
Severity Score:1.0
Description in Drug Labeling: View Full Labeling: SPL in DailyMed | PDF
- CLINICAL PHARMACOLOGY
- Pharmacodynamics
- Cardiac Electrophysiology
- In a randomized, double-blind placebo- and (open-label) moxifloxacin-controlled 4-period crossover study, 56 healthy subjects were administered methylnaltrexone bromide 0.3 mg/kg and methylnaltrexone bromide 0.64 mg/kg by intravenous infusion over 20 minutes (RELISTOR is not approved for intravenous use), placebo, and a single oral dose of moxifloxacin. At a dose approximately 4.3 times the maximum recommended dose (7.5 times the mean peak plasma concentration for RELISTOR injection and 22 times the peak plasma concentration for RELISTOR tablets), methylnaltrexone does not prolong the QTc interval to any clinically relevant extent.
- NONCLINICAL TOXICOLOGY
- Animal Toxicology and/or Pharmacology
- In an in vitro human cardiac potassium ion channel (hERG) assay, methylnaltrexone caused concentration-dependent inhibition of hERG current (1%, 12%, 13% and 40% inhibition at 30, 100, 300 and 1000 micromolar concentrations, respectively). Methylnaltrexone had a hERG IC50 of more than 1000 micromolar. In isolated dog Purkinje fibers, methylnaltrexone caused prolongations in action potential duration (APD). The highest tested concentration (10 micromolar) in the dog Purkinje fiber study was about 18 and 37 times the Cmax at human subcutaneous doses of 0.3 and 0.15 mg/kg, respectively. In isolated rabbit Purkinje fibers, methylnaltrexone (up to 100 micromolar) did not have an effect on APD, compared to vehicle control. The highest methylnaltrexone concentration (100 micromolar) tested was about 186 and 373 times the human Cmax at subcutaneous doses of 0.3 and 0.15 mg/kg, respectively. In anesthetized dogs, methylnaltrexone bromide caused decreases in blood pressure, heart rate, cardiac output, left ventricular pressure, left ventricular end diastolic pressure, and +dP/dt at 1 mg/kg or more. In conscious dogs, methylnaltrexone bromide caused a dose-related increase in QTc interval. After a single intravenous dosage of 20 mg/kg to beagle dogs, predicted Cmax and AUC values were approximately 482 and 144 times, respectively, the exposure at human subcutaneous dose of 0.15 mg/kg and 241 times and 66 times, respectively, the exposure at a human subcutaneous dose of 0.3 mg/kg. In conscious guinea pigs, methylnaltrexone bromide caused mild prolongation of QTc (4% over baseline) at 20 mg/kg, intravenous. A thorough QTc assessment was conducted in humans [see Clinical Pharmacology (12.2)].
- In juvenile rats administered intravenous methylnaltrexone bromide for 13 weeks, adverse clinical signs such as convulsions, tremors and labored breathing occurred at dosages of 3 and 10 mg/kg/day (about 2.4 and 8 times, respectively, the subcutaneous MRHD of 12 mg/day; about 0.06 and 0.22 times, respectively, the oral MRHD of 450 mg/day). Similar adverse clinical signs were seen in adult rats at 20 mg/kg/day (about 16 times the subcutaneous MRHD of 12 mg/day; about 0.43 times the oral MRHD of 450 mg/day). Juvenile rats were found to be more sensitive to the toxicity of methylnaltrexone bromide when compared to adults. The no observed adverse effect levels (NOAELs) in juvenile and adult rats were 1 and 5 mg/kg/day, respectively (about 0.8 and 4 times, respectively, the subcutaneous MRHD of 12 mg/day; about 0.02 and 0.11 times, respectively, the oral MRHD of 450 mg/day).
- Juvenile dogs administered intravenous methylnaltrexone bromide for 13 weeks had a toxicity profile similar to adult dogs. Following intravenous administration of methylnaltrexone bromide for 13 weeks, decreased heart rate (13.2% reduction compared to pre-dose) in juvenile dogs and prolonged QTc interval in juvenile (9.6% compared to control) and adult (up to 15% compared to control) dogs occurred at 20 mg/kg/day (about 54 times the subcutaneous MRHD of 12 mg/day; about 1.5 times the oral MRHD of 450 mg/day). Clinical signs consistent with effects on the CNS (including tremors and decreased activity) occurred in both juvenile and adult dogs. The NOAELs in juvenile and adult dogs were 5 mg/kg/day (about 14 times the subcutaneous MRHD of 12 mg/day; about 0.4 times the oral MRHD of 450 mg/day).
Postmarketing Surveillance
Contingency Table:
Current Drug
Other Drugs
QT Prolongation
0
24092
Other ADRs
1781
38379806
Odds Ratio = 0.0
Drug Property Information
ATC Code(s):
- A06AH01 - methylnaltrexone bromide
- A06AH0 -
- A06AH -
- A06A - LAXATIVES
- A06 - LAXATIVES
- A - ALIMENTARY TRACT AND METABOLISM
Active Ingredient:METHYLNALTREXONE BROMIDE
Active Ingredient UNII:RFO6IL3D3M
Drugbank ID:DB06800
PubChem Compound:16089915
CTD ID:C032257
CAS Number:916055-93-1
Dosage Form(s):injection, solution; tablet
Route(s) Of Administrator:oral; subcutaneous
Daily Dose:
- 6.0 mg/day A06AH01
Chemical Structure: 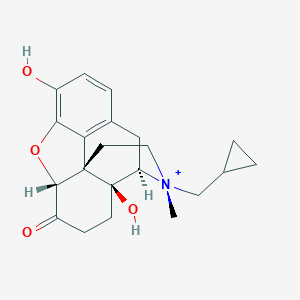

SMILE Code:
C[N@+]1(CC2CC2)CC[C@]23[C@H]4OC5=C(O)C=CC(C[C@@H]1[C@]2(O)CCC4=O)=C35
C[N@+]1(CC2CC2)CC[C@]23[C@H]4OC5=C(O)C=CC(C[C@@H]1[C@]2(O)CCC4=O)=C35
Reference
N/A
Disclaimer:
The content of this database of QT prolongation is intended for educational and scientific research purposes only. It is not intended as a substitute for professional medical advice, diagnosis or treatment.
Any mention of commercial products is for clarification and not intended as endorsement.