Search for drugs:
Typing the drug name to query
PALIPERIDONE PALMITATE
DIR Classification
Classification:Most-DIQT concern
Severity Score:4.0
Description in Drug Labeling: View Full Labeling: SPL in DailyMed | PDF
- WARNINGS AND PRECAUTIONS
- QT Prolongation
- Paliperidone causes a modest increase in the corrected QT (QTc) interval. The use of paliperidone should be avoided in combination with other drugs that are known to prolong QTc including Class 1A (e.g., quinidine, procainamide) or Class III (e.g., amiodarone, sotalol) antiarrhythmic medications, antipsychotic medications (e.g., chlorpromazine, thioridazine), antibiotics (e.g., gatifloxacin, moxifloxacin), or any other class of medications known to prolong the QTc interval. Paliperidone should also be avoided in patients with congenital long QT syndrome and in patients with a history of cardiac arrhythmias.
- Certain circumstances may increase the risk of the occurrence of Torsades de pointes and/or sudden death in association with the use of drugs that prolong the QTc interval, including (1) bradycardia; (2) hypokalemia or hypomagnesemia; (3) concomitant use of other drugs that prolong the QTc interval; and (4) presence of congenital prolongation of the QT interval.
- The effects of paliperidone on the QT interval were evaluated in a double-blind, active-controlled (moxifloxacin 400 mg single dose), multicenter Thorough QT study with oral paliperidone in adult patients, and in four fixed-dose efficacy studies and one maintenance study of the 1-month paliperidone palmitate injectable product.
- In the Thorough QT study (n=141), the 8 mg dose of immediate-release oral paliperidone (n=50) showed a mean placebo-subtracted increase from baseline in QTcLD (QT interval corrected for heart rate using the population specified linear derived method) of 12.3 msec (90% CI: 8.9; 15.6) on day 8 at 1.5 hours post-dose. The mean steady-state peak plasma concentration for this 8 mg dose of paliperidone immediate release (Cmax ss=113 ng/mL) was approximately 2-fold the exposure with the maximum recommended 819 mg dose of INVEGA TRINZA® administered in the deltoid muscle (predicted median Cmax ss=56 ng/mL). In this same study, a 4 mg dose of the immediate-release oral formulation of paliperidone, for which Cmax ss=35 ng/mL, showed an increased placebo-subtracted QTcLD of 6.8 msec (90% CI: 3.6; 10.1) on day 2 at 1.5 hours post-dose.
- In the four fixed-dose efficacy studies of the 1-month paliperidone palmitate injectable product, no subject had a change in QTcLD exceeding 60 msec and no subject had a QTcLD value of > 500 msec at any time point. In the maintenance study, no subject had a QTcLD change > 60 msec, and one subject had a QTcLD value of 507 msec (Bazett's QT corrected interval [QTcB] value of 483 msec); this latter subject also had a heart rate of 45 beats per minute.
- In the long-term maintenance trial of INVEGA TRINZA® in subjects with schizophrenia, an increase in QTcLD exceeding 60 msec was observed in 1 subject (< 1%) in the open-label phase, no subject had an increase in QTcLD exceeding 60 msec after treatment with INVEGA TRINZA® in the double-blind phase, and no subject had a QTcLD value of > 480 msec at any point in the study.
- OVERDOSAGE
- Human Experience
- No cases of overdose were reported in premarketing studies with paliperidone palmitate injection. Because INVEGA TRINZA® is to be administered by health care professionals, the potential for overdosage by patients is low.
- While experience with paliperidone overdose is limited, among the few cases of overdose reported in premarketing trials with oral paliperidone, the highest estimated ingestion was 405 mg. Observed signs and symptoms included extrapyramidal symptoms and gait unsteadiness. Other potential signs and symptoms include those resulting from an exaggeration of paliperidone's known pharmacological effects, i.e., drowsiness and sedation, tachycardia and hypotension, and QT prolongation. Torsades de pointes and ventricular fibrillation have been reported in a patient in the setting of overdose with oral paliperidone.
- Paliperidone is the major active metabolite of risperidone. Overdose experience reported with risperidone can be found in the OVERDOSAGE section of the risperidone package insert.
- ADVERSE REACTIONS
- The following are discussed in more detail in other sections of the labeling:
- Increased mortality in elderly patients with dementia-related psychosis [see BOXED WARNING and WARNINGS AND PRECAUTIONS (5.1)]
- Cerebrovascular adverse reactions, including stroke, in elderly patients with dementia-related psychosis [see WARNINGS AND PRECAUTIONS (5.2)]
- Neuroleptic malignant syndrome [see WARNINGS AND PRECAUTIONS (5.3)]
- QT prolongation [see WARNINGS AND PRECAUTIONS (5.4)]
- Tardive dyskinesia [see WARNINGS AND PRECAUTIONS (5.5)]
- Metabolic changes [see WARNINGS AND PRECAUTIONS (5.6)]
- Orthostatic hypotension and syncope [see WARNINGS AND PRECAUTIONS (5.7)]
- Falls [see WARNINGS AND PRECAUTIONS (5.8)]
- Leukopenia, neutropenia, and agranulocytosis [see WARNINGS AND PRECAUTIONS (5.9)]
- Hyperprolactinemia [see WARNINGS AND PRECAUTIONS (5.10)]
- Potential for cognitive and motor impairment [see WARNINGS AND PRECAUTIONS (5.11)]
- Seizures [see WARNINGS AND PRECAUTIONS (5.12)]
- Dysphagia [see WARNINGS AND PRECAUTIONS (5.13)]
- Priapism [see WARNINGS AND PRECAUTIONS (5.14)]
- Disruption of body temperature regulation [see WARNINGS AND PRECAUTIONS (5.15)]
Postmarketing Surveillance
Contingency Table:
Current Drug
Other Drugs
QT Prolongation
80
24012
Other ADRs
62021
38319566
Odds Ratio = 2.059
Drug Property Information
ATC Code(s):
- N05AX13 - paliperidone palmitate
- N05AX - Other antipsychotics
- N05A - ANTIPSYCHOTICS
- N05 - PSYCHOLEPTICS
- N - NERVOUS SYSTEM
Active Ingredient:paliperidone palmitate
Active Ingredient UNII:R8P8USM8FR
Drugbank ID:DB01267
PubChem Compound:115237
CTD ID:D000068882
PharmGKB:PA163518919
CAS Number:144598-75-4
Dosage Form(s):injection, suspension, extended release
Route(s) Of Administrator:intramuscular
Daily Dose:
- 6.0 mg/day N05AX13
Chemical Structure: 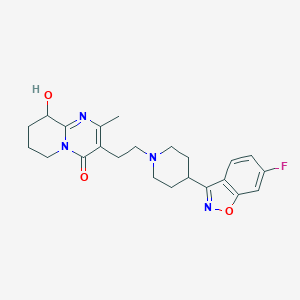

SMILE Code:
CC1=C(CCN2CCC(CC2)C2=NOC3=C2C=CC(F)=C3)C(=O)N2CCCC(O)C2=N1
CC1=C(CCN2CCC(CC2)C2=NOC3=C2C=CC(F)=C3)C(=O)N2CCCC(O)C2=N1
Reference
1: Need for Bioequivalence Standards that Reflect the Clinical Importance of the Complex Pharmacokinetics of Paliperidone Palmitate Long-Acting Injectable Suspension.
[Procyshyn Ric M,Lamoure Joel W,Katzman Martin A,Skinner Pamela L,Sherman Stephen E]J Pharm Pharm Sci,2019;22(1):548-566. PMID: 31730504
2: Analysis of proarrhythmic potential of an atypical antipsychotic drug paliperidone in the halothane-anesthetized dogs.
[Chiba Koki,Wada Takeshi,Nakamura Yuji,Cao Xin,Hagiwara-Nagasawa Mihoko,Izumi-Nakaseko Hiroko,Ando Kentaro,Tanaka Koichiro,Naito Atsuhiko T,Sugiyama Atsushi]J Pharmacol Sci,2017 Aug;134(4):239-246. PMID: 28844424
3: Effect of lipid emulsion infusion on paliperidone pharmacokinetics in the acute overdose rat model: A potential emergency treatment for paliperidone intoxication.
[Enokiya Tomoyuki,Zhang Erquan,Ikemura Kenji,Muraki Yuichi,Iwashita Yoshiaki,Iwamoto Takuya,Imai Hiroshi,Maruyama Kazuo,Okuda Masahiro]Eur J Pharm Sci,2017 Nov 15;109:217-222. PMID: 28821438
4: In vivo analysis of torsadogenic potential of an antipsychotic drug paliperidone using the acute atrioventricular block rabbit as a proarrhythmia model.
[Hagiwara Mihoko,Kambayashi Ryuichi,Aimoto Megumi,Nagasawa Yoshinobu,Takahara Akira]J Pharmacol Sci,2016 Sep;132(1):48-54. PMID: 27262905
5: Risk of cardiovascular morbidity with risperidone or paliperidone treatment: analysis of 64 randomized, double-blind trials.
[Gopal Srihari,Hough David,Karcher Keith,Nuamah Isaac,Palumbo Joseph,Berlin Jesse A,Baseman Alan,Xu Yimei,Kent Justine]J Clin Psychopharmacol,2013 Apr;33(2):157-61. PMID: 23422378
6: QT prolongation of the antipsychotic risperidone is predominantly related to its 9-hydroxy metabolite paliperidone.
[Suzuki Yutaro,Fukui Naoki,Watanabe Junzo,Ono Shin,Sugai Takuro,Tsuneyama Nobuto,Saito Mami,Inoue Yoshimasa,Someya Toshiyuki]Hum Psychopharmacol,2012 Jan;27(1):39-42. PMID: 22144033
7: In vivo and in vitro myocardial binding of risperidone and 9-hydroxyrisperidone.
[Titier Karine,Déridet Evelyne,Moore Nicholas]Toxicol Appl Pharmacol,2002 Apr 15;180(2):145-9. PMID: 11969382
Disclaimer:
The content of this database of QT prolongation is intended for educational and scientific research purposes only. It is not intended as a substitute for professional medical advice, diagnosis or treatment.
Any mention of commercial products is for clarification and not intended as endorsement.