Search for drugs:
Typing the drug name to query
COBIMETINIB
DIR Classification
Classification:Ambiguous
Severity Score:1.0
Description in Drug Labeling: View Full Labeling: SPL in DailyMed | PDF
- CLINICAL PHARMACOLOGY
- Pharmacodynamics
- Cardiac Electrophysiology
- Clinically relevant QT prolongation has been reported with vemurafenib, further QTc prolongation was not observed when cobimetinib 60 mg daily was co-administered with vemurafenib. Monitor ECG and electrolytes before initiating treatment and routinely during treatment with cobimetinib, when administered with vemurafenib. Review the Full Prescribing Information for vemurafenib for details.
Postmarketing Surveillance
Contingency Table:
Current Drug
Other Drugs
QT Prolongation
12
24080
Other ADRs
3225
38378362
Odds Ratio = 5.931
Drug Property Information
ATC Code(s):
- L01EE02 - cobimetinib
- L01EE0 -
- L01EE -
- L01E -
- L01 - ANTINEOPLASTIC AGENTS
- L - ANTINEOPLASTIC AND IMMUNOMODULATING AGENTS
Active Ingredient:COBIMETINIB FUMARATE
Active Ingredient UNII:6EXI96H8SV
Drugbank ID:DB05239
PubChem Compound:16222096
CTD ID: C574276
PharmGKB:PA166160044
CAS Number:934660-93-2
Dosage Form(s):tablet, film coated
Route(s) Of Administrator:oral
Daily Dose:
Chemical Structure: 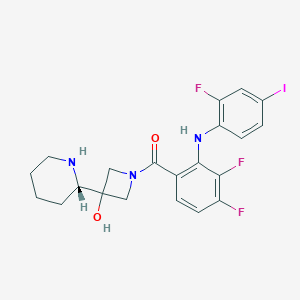

SMILE Code:
OC1(CN(C1)C(=O)C1=C(NC2=C(F)C=C(I)C=C2)C(F)=C(F)C=C1)[C@@H]1CCCCN1
OC1(CN(C1)C(=O)C1=C(NC2=C(F)C=C(I)C=C2)C(F)=C(F)C=C1)[C@@H]1CCCCN1
Reference
1: Tolerability of BRAF/MEK inhibitor combinations: adverse event evaluation and management.
[Heinzerling Lucie,Eigentler Thomas K,Fluck Michael,Hassel Jessica C,Heller-Schenck Daniela,Leipe Jan,Pauschinger Matthias,Vogel Arndt,Zimmer Lisa,Gutzmer Ralf]ESMO Open,2019 May 23;4(3):e000491. PMID: 31231568
Disclaimer:
The content of this database of QT prolongation is intended for educational and scientific research purposes only. It is not intended as a substitute for professional medical advice, diagnosis or treatment.
Any mention of commercial products is for clarification and not intended as endorsement.