Search for drugs:
Typing the drug name to query
IBUTILIDE FUMARATE
DIR Classification
Classification:Most-DIQT concern
Severity Score:5.0
Description in Drug Labeling: View Full Labeling: SPL in DailyMed | PDF
- BOXED WARNING
- LIFE-THREATENING ARRHYTHMIAS-APPROPRIATE TREATMENT ENVIRONMENT
- Ibutilide fumarate injection can cause potentially fatal arrhythmias, particularly sustained polymorphic ventricular tachycardia, usually in association with QT prolongation (torsades de pointes), but sometimes without documented QT prolongation. In registration studies, these arrhythmias, which require cardioversion, occurred in 1.7% of treated patients during, or within a number of hours of, use of ibutilide fumarate injection. These arrhythmias can be reversed if treated promptly (see WARNINGS, PROARRHYTHMIA). It is essential that ibutilide fumarate injection be administered in a setting of continuous ECG monitoring and by personnel trained in identification and treatment of acute ventricular arrhythmias, particularly polymorphic ventricular tachycardia. Patients with atrial fibrillation of more than 2 to 3 days' duration must be adequately anticoagulated, generally for at least 2 weeks.
- WARNINGS
- Like other antiarrhythmic agents, ibutilide fumarate injection can induce or worsen ventricular arrhythmias in some patients. This may have potentially fatal consequences. Torsades de pointes, a polymorphic ventricular tachycardia that develops in the setting of a prolonged QT interval, may occur because of the effect ibutilide fumarate injection has on cardiac repolarization, but ibutilide fumarate injection can also cause polymorphic VT in the absence of excessive prolongation of the QT interval. In general, with drugs that prolong the QT interval, the risk of torsades de pointes is thought to increase progressively as the QT interval is prolonged and may be worsened with bradycardia, a varying heart rate, and hypokalemia. In clinical trials conducted in patients with atrial fibrillation and atrial flutter, those with QTc intervals >440 msec were not usually allowed to participate, and serum potassium had to be above 4.0 mEq/L. Although change in QTc was dose dependent for ibutilide, there was no clear relationship between risk of serious proarrhythmia and dose in clinical studies, possibly due to the small number of events. In clinical trials of intravenous ibutilide, patients with a history of congestive heart failure (CHF) or low left ventricular ejection fraction appeared to have a higher incidence of sustained polymorphic ventricular tachycardia (VT), than those without such underlying conditions; for sustained polymorphic VT the rate was 5.4% in patients with a history of CHF and 0.8% without it. There was also a suggestion that women had a higher risk of proarrhythmia, but the sex difference was not observed in all studies and was most prominent for nonsustained ventricular tachycardia. The incidence of sustained ventricular arrhythmias was similar in male (1.8%) and female (1.5%) patients, possibly due to the small number of events. Ibutilide fumarate injection is not recommended in patients who have previously demonstrated polymorphic ventricular tachycardia (e.g., torsades de pointes).
- PRECAUTIONS
- Other drugs that prolong the QT interval
- The potential for proarrhythmia may increase with the administration of ibutilide fumarate injection to patients who are being treated with drugs that prolong the QT interval, such as phenothiazines, tricyclic antidepressants, tetracyclic antidepressants, and certain antihistamine drugs (H1 receptor antagonists).
- DOSAGE AND ADMINISTRATION
- The recommended dose based on controlled trials (see CLINICAL PHARMACOLOGY: CLINICAL STUDIES) is outlined in the Table below. Ibutilide infusion should be stopped as soon as the presenting arrhythmia is terminated or in the event of sustained or nonsustained ventricular tachycardia, or marked prolongation of QT or QTc.
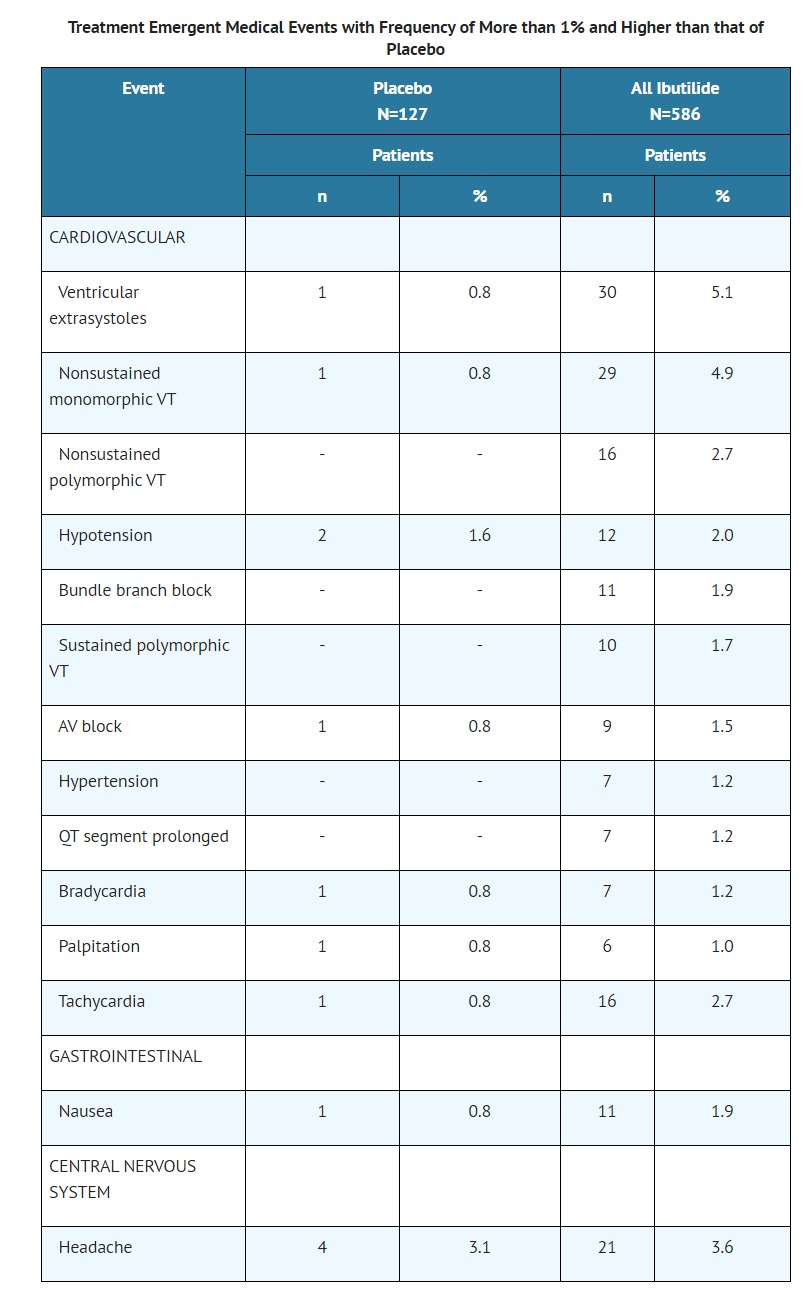
- CLINICAL PHARMACOLOGY
- Electrophysiologic Effects
- Ibutilide fumarate injection produces mild slowing of the sinus rate and atrioventricular conduction. Ibutilide fumarate injection produces no clinically significant effect on QRS duration at intravenous doses up to 0.03 mg/kg administered over a 10-minute period. Although there is no established relationship between plasma concentration and antiarrhythmic effect, ibutilide fumarate injection produces dose-related prolongation of the QT interval, which is thought to be associated with its antiarrhythmic activity. (See WARNINGS for relationship between QTc prolongation and torsades de pointes-type arrhythmias.) In a study in healthy volunteers, intravenous infusions of ibutilide fumarate injection resulted in prolongation of the QT interval that was directly correlated with ibutilide plasma concentration during and after 10-minute and 8-hour infusions. A steep ibutilide concentration/response (QT prolongation) relationship was shown. The maximum effect was a function of both the dose of ibutilide fumarate injection and the infusion rate.
Postmarketing Surveillance
Contingency Table:
Current Drug
Other Drugs
QT Prolongation
7
24085
Other ADRs
50
38381537
Odds Ratio = 223.103
Drug Property Information
ATC Code(s):
- C01BD05 - ibutilide fumarate
- C01BD - "Antiarrhythmics, class III"
- C01B - "ANTIARRHYTHMICS, CLASS I AND III"
- C01 - CARDIAC THERAPY
- C - CARDIOVASCULAR SYSTEM
Active Ingredient:IBUTILIDE FUMARATE
Active Ingredient UNII:9L5X4M5L6I
Drugbank ID:DB00308
PubChem Compound:60753
CTD ID:C067192
PharmGKB:PA449958
CAS Number:122647-31-8
Dosage Form(s):injection, solution
Route(s) Of Administrator:intravenous
Daily Dose:
- 1.0 mg/day C01BD05
Chemical Structure: 

SMILE Code:
CCCCCCCN(CC)CCCC(O)C1=CC=C(NS(C)(=O)=O)C=C1
CCCCCCCN(CC)CCCC(O)C1=CC=C(NS(C)(=O)=O)C=C1
Reference
1: Efficacy and safety of ibutilide for the conversion of monomorphic atrial tachycardia.
[Eidher Ulrike,Freihoff Fritz,Kaltenbrunner Wilhelm,Steinbach Konrad]Pacing Clin Electrophysiol,2006 Apr;29(4):358-62. PMID: 16650262
2: Electrophysiology and pharmacology of ibutilide.
[Naccarelli G V,Lee K S,Gibson J K,VanderLugt J]Am J Cardiol,1996 Oct 17;78(8A):12-6. PMID: 8903270
Disclaimer:
The content of this database of QT prolongation is intended for educational and scientific research purposes only. It is not intended as a substitute for professional medical advice, diagnosis or treatment.
Any mention of commercial products is for clarification and not intended as endorsement.