Search for drugs:
Typing the drug name to query
NIRAPARIB
DIR Classification
Classification:Ambiguous
Severity Score:1.0
Description in Drug Labeling: View Full Labeling: SPL in DailyMed | PDF
- CLINICAL PHARMACOLOGY
- Pharmacodynamics
- Cardiac Electrophysiology
- The potential for QTc prolongation with niraparib was evaluated in a randomized, placebo-controlled trial in cancer patients (367 patients on niraparib and 179 patients on placebo). No large changes in the mean QTc interval (>20 ms) were detected in the trial following the treatment of niraparib 300 mg once daily.
Postmarketing Surveillance
Contingency Table:
Current Drug
Other Drugs
QT Prolongation
3
24089
Other ADRs
60968
38320619
Odds Ratio = 0.079
Drug Property Information
ATC Code(s):
- L01XK02 - niraparib
- L01XK -
- L01X - OTHER ANTINEOPLASTIC AGENTS
- L01 - ANTINEOPLASTIC AGENTS
- L - ANTINEOPLASTIC AND IMMUNOMODULATING AGENTS
Active Ingredient:niraparib
Active Ingredient UNII:HMC2H89N35
Drugbank ID:DB11793
PubChem Compound:24958200
CTD ID: C545685
PharmGKB:PA166131610
CAS Number:1038915-60-4
Dosage Form(s):capsule
Route(s) Of Administrator:oral
Daily Dose:
Chemical Structure: 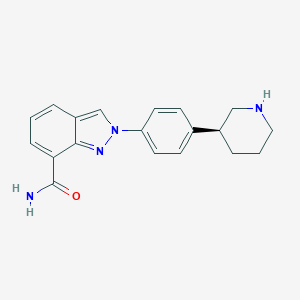

SMILE Code:
NC(=O)C1=CC=CC2=CN(N=C12)C1=CC=C(C=C1)[C@@H]1CCCNC1
NC(=O)C1=CC=CC2=CN(N=C12)C1=CC=C(C=C1)[C@@H]1CCCNC1
Reference
1: Effect of niraparib on cardiac repolarization in patients with platinum-sensitive, recurrent epithelial ovarian, fallopian tube, and primary peritoneal cancer.
[Moore Kathleen,Chan John K,Secord Angeles Alvarez,Patel Manish R,Callahan Timothy,Guo Wei,Zhang Zhi-Yi]Cancer Chemother Pharmacol,2019 Apr;83(4):717-726. PMID: 30680521
Disclaimer:
The content of this database of QT prolongation is intended for educational and scientific research purposes only. It is not intended as a substitute for professional medical advice, diagnosis or treatment.
Any mention of commercial products is for clarification and not intended as endorsement.