Search for drugs:
Typing the drug name to query
TELAVANCIN HYDROCHLORIDE
DIR Classification
Classification:Moderate-DIQT concern
Severity Score:3.0
Description in Drug Labeling: View Full Labeling: SPL in DailyMed | PDF
- WARNINGS AND PRECAUTIONS
- QTc Prolongation
- In a study involving healthy volunteers, doses of 7.5 and 15 mg/kg of VIBATIV prolonged the QTc interval [see Clinical Pharmacology (12.2)]. Caution is warranted when prescribing VIBATIV to patients taking drugs known to prolong the QT interval. Patients with congenital long QT syndrome, known prolongation of the QTc interval, uncompensated heart failure, or severe left ventricular hypertrophy were not included in clinical trials of VIBATIV. Use of VIBATIV should be avoided in patients with these conditions.
- Serious adverse events were reported in 31% of patients treated with VIBATIV and 26% of patients who received vancomycin. Treatment discontinuations due to adverse events occurred in 8% (60/751) of patients who received VIBATIV, the most common events being acute renal failure and electrocardiogram QTc interval prolonged (~1% each). Treatment discontinuations due to adverse events occurred in 5% (40/752) of vancomycin-patients, the most common events being septic shock and multi-organ failure (<1%).
- CLINICAL PHARMACOLOGY
- Pharmacodynamics
- Cardiac Electrophysiology
- The effect of telavancin on cardiac repolarization was assessed in a randomized, double-blind, multiple-dose, positive-controlled, and placebo-controlled, parallel study (n=160). Healthy subjects received VIBATIV 7.5 mg/kg, VIBATIV 15 mg/kg, positive control, or placebo infused over 60 minutes once daily for 3 days. Based on interpolation of the data from VIBATIV 7.5 mg/kg and 15 mg/kg, the mean maximum baseline-corrected, placebo-corrected QTc prolongation at the end of infusion was estimated to be 12-15 msec for VIBATIV 10 mg/kg and 22 msec for the positive control (Table 7). By 1 hour after infusion the maximum QTc prolongation was 6-9 msec for VIBATIV and 15 msec for the positive control.
- ECGs were performed prior to and during the treatment period in patients receiving VIBATIV 10 mg/kg in 3 cSSSI studies to monitor QTc intervals. In these trials, 214 of 1029 (21%) patients allocated to treatment with VIBATIV and 164 of 1033 (16%) allocated to vancomycin received concomitant medications known to prolong the QTc interval and known to be associated with definite or possible risk of torsades de pointes. The incidence of QTc prolongation >60 msec was 1.5% (15 patients) in the VIBATIV group and 0.6% (6 patients) in the vancomycin group. Nine of the 15 VIBATIV patients received concomitant medications known to prolong the QTc interval and definitely or possibly associated with a risk of torsades de pointes, compared with 1 of the 6 patients who received vancomycin. A similar number of patients in each treatment group (<1%) who did not receive a concomitant medication known to prolong the QTc interval experienced a prolongation >60 msec from baseline. In a separate analysis, 1 patient in the VIBATIV group and 2 patients in the vancomycin group experienced QTc >500 msec. No cardiac adverse events were ascribed to prolongation of the QTc interval. In the Phase 3 HABP/VABP studies, the incidence of QTc prolongation >60 msec or mean value >500 msec was 8% (52 patients) in the telavancin group and 7% (48 patients) in the vancomycin group.
- MEDICATION GUIDE
- VIBATIV may cause serious side effects, including:
- Problems with the electrical system of your heart (QTc prolongation). Tell your healthcare provider right away if you have a change in your heartbeat such as a fast or irregular heartbeat or if you had a fainting episode.
Postmarketing Surveillance
Contingency Table:
Current Drug
Other Drugs
QT Prolongation
1
24091
Other ADRs
352
38381235
Odds Ratio = 4.527
Drug Property Information
ATC Code(s):
- J01XA03 - telavancin hydrochloride
- J01XA - Glycopeptide antibacterials
- J01X - OTHER ANTIBACTERIALS
- J01 - ANTIBACTERIALS FOR SYSTEMIC USE
- J - ANTIINFECTIVES FOR SYSTEMIC USE
Active Ingredient:telavancin hydrochloride
Active Ingredient UNII:0701472ZG0
Drugbank ID:DB06402
PubChem Compound:3081362
CTD ID:C487637
CAS Number:372151-71-8
Dosage Form(s):injection, powder, lyophilized, for solution
Route(s) Of Administrator:intravenous
Daily Dose:
Chemical Structure: 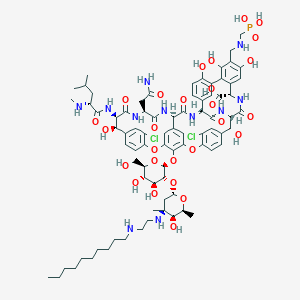

SMILE Code:
CCCCCCCCCCNCCN[C@@]1(C)C[C@H](O[C@@H]2[C@@H](O)[C@H](O)[C@@H](CO)O[C@H]2OC2=C3OC4=CC=C(C=C4Cl)[C@@H](O)[C@@H](NC(=O)[C@@H](CC(C)C)NC)C(=O)N[C@@H](CC(N)=O)C(=O)N[C@@H]4C(C=C2OC2=C(Cl)C=C(C=C2)[C@@H](O)[C@@H]2NC(=O)[C@H](NC4=O)C4=CC(=C(O)C=C4)C4=C(O)C(CNCP(O)(O)=O)=C(O)C=C4[C@H](NC2=O)C(O)=O)=C3)O[C@@H](C)[C@H]1O
CCCCCCCCCCNCCN[C@@]1(C)C[C@H](O[C@@H]2[C@@H](O)[C@H](O)[C@@H](CO)O[C@H]2OC2=C3OC4=CC=C(C=C4Cl)[C@@H](O)[C@@H](NC(=O)[C@@H](CC(C)C)NC)C(=O)N[C@@H](CC(N)=O)C(=O)N[C@@H]4C(C=C2OC2=C(Cl)C=C(C=C2)[C@@H](O)[C@@H]2NC(=O)[C@H](NC4=O)C4=CC(=C(O)C=C4)C4=C(O)C(CNCP(O)(O)=O)=C(O)C=C4[C@H](NC2=O)C(O)=O)=C3)O[C@@H](C)[C@H]1O
Reference
N/A
Disclaimer:
The content of this database of QT prolongation is intended for educational and scientific research purposes only. It is not intended as a substitute for professional medical advice, diagnosis or treatment.
Any mention of commercial products is for clarification and not intended as endorsement.