Search for drugs:
Typing the drug name to query
ABEMACICLIB
DIR Classification
Classification:Ambiguous
Severity Score:1.0
Description in Drug Labeling: View Full Labeling: SPL in DailyMed | PDF
- ADVERSE REACTIONS
- Clinical Studies Experience
- Ten patients (8%) discontinued study treatment from adverse reactions due to (1 patient each) abdominal pain, arterial thrombosis, aspartate aminotransferase (AST) increased, blood creatinine increased, chronic kidney disease, diarrhea, ECG QT prolonged, fatigue, hip fracture, and lymphopenia. Forty-nine percent of patients had dose reductions due to an adverse reaction. The most frequent adverse reactions that led to dose reductions were diarrhea (20%), neutropenia (11%), and fatigue (9%).
- CLINICAL PHARMACOLOGY
- Pharmacodynamics
- Cardiac Electrophysiology
- Based on evaluation of the QTc interval in patients and in a healthy volunteer study, abemaciclib did not cause large mean increases (i.e., 20 ms) in the QTc interval.
Postmarketing Surveillance
Contingency Table:
Current Drug
Other Drugs
QT Prolongation
0
24092
Other ADRs
8195
38373392
Odds Ratio = 0.0
Drug Property Information
ATC Code(s):
- L01EF03 - abemaciclib
- L01EF -
- L01E -
- L01 - ANTINEOPLASTIC AGENTS
- L - ANTINEOPLASTIC AND IMMUNOMODULATING AGENTS
Active Ingredient:abemaciclib
Active Ingredient UNII:60UAB198HK
Drugbank ID:DB12001
PubChem Compound:46220502
CTD ID: C000590451
PharmGKB:PA166153471
CAS Number:1231929-97-7
Dosage Form(s):tablet
Route(s) Of Administrator:oral
Daily Dose:
Chemical Structure: 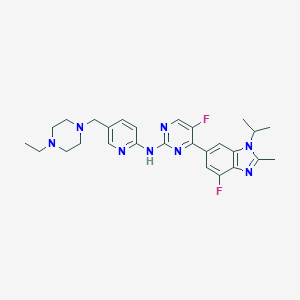

SMILE Code:
CCN1CCN(CC2=CC=C(NC3=NC=C(F)C(=N3)C3=CC(F)=C4N=C(C)N(C(C)C)C4=C3)N=C2)CC1
CCN1CCN(CC2=CC=C(NC3=NC=C(F)C(=N3)C3=CC(F)=C4N=C(C)N(C(C)C)C4=C3)N=C2)CC1
Reference
1: CDK4/6 inhibitors in breast cancer: differences in toxicity profiles and impact on agent choice. A systematic review and meta-analysis.
[Onesti Concetta E,Jerusalem Guy]Expert Rev Anticancer Ther,2021 Mar;21(3):283-298. PMID: 33233970
2: Clinical Pharmacokinetics and Pharmacodynamics of the Cyclin-Dependent Kinase 4 and 6 Inhibitors Palbociclib, Ribociclib, and Abemaciclib.
[Groenland Stefanie L,Martínez-Chávez Alejandra,van Dongen Marloes G J,Beijnen Jos H,Schinkel Alfred H,Huitema Alwin D R,Steeghs Neeltje]Clin Pharmacokinet,2020 Dec;59(12):1501-1520. PMID: 33029704
3: Comparative efficacy of palbociclib, ribociclib and abemaciclib for ER+ metastatic breast cancer: an adjusted indirect analysis of randomized controlled trials.
[Petrelli Fausto,Ghidini Antonio,Pedersini Rebecca,Cabiddu Mary,Borgonovo Karen,Parati Maria Chiara,Ghilardi Mara,Amoroso Vito,Berruti Alfredo,Barni Sandro]Breast Cancer Res Treat,2019 Apr;174(3):597-604. PMID: 30659432
4: Management of adverse events during cyclin-dependent kinase 4/6 (CDK4/6) inhibitor-based treatment in breast cancer.
[Thill Marc,Schmidt Marcus]Ther Adv Med Oncol,2018 Sep 3;10:1758835918793326. PMID: 30202447
Disclaimer:
The content of this database of QT prolongation is intended for educational and scientific research purposes only. It is not intended as a substitute for professional medical advice, diagnosis or treatment.
Any mention of commercial products is for clarification and not intended as endorsement.