Search for drugs:
Typing the drug name to query
MONTELUKAST
DIR Classification
Classification:Ambiguous
Severity Score:1.0
Description in Drug Labeling: View Full Labeling: SPL in DailyMed | PDF
- CLINICAL PHARMACOLOGY
- Pharmacokinetics
- Drug-Drug Interactions
- Oral Contraceptives, Terfenadine, Digoxin and Warfarin: In drug interaction studies, the recommended clinical dose of montelukast did not have clinically important effects on the pharmacokinetics of the following drugs: oral contraceptives (norethindrone 1 mg/ethinyl estradiol 35 mcg), terfenadine, digoxin and warfarin. Montelukast at doses of ≥100 mg daily dosed to pharmacokinetic steady-state did not significantly alter the plasma concentrations of either component of an oral contraceptive containing norethindrone 1 mg/ethinyl estradiol 35 mcg. Montelukast at a dose of 10 mg once daily dosed to pharmacokinetic steady-state did not change the plasma concentration profile of terfenadine (a substrate of CYP3A4) or fexofenadine, the carboxylated metabolite, and did not prolong the QTc interval following co-administration with terfenadine 60 mg twice daily; did not change the pharmacokinetic profile or urinary excretion of immunoreactive digoxin; did not change the pharmacokinetic profile of warfarin (primarily a substrate of CYP2C9, 3A4 and 1A2) or influence the effect of a single 30 mg oral dose of warfarin on prothrombin time or the International Normalized Ratio (INR).
Postmarketing Surveillance
Contingency Table:
Current Drug
Other Drugs
QT Prolongation
18
24074
Other ADRs
61310
38320277
Odds Ratio = 0.468
Drug Property Information
ATC Code(s):
- R03DC03 - montelukast
- R03DC - Leukotriene receptor antagonists
- R03D - OTHER SYSTEMIC DRUGS FOR OBSTRUCTIVE AIRWAY DISEASES
- R03 - DRUGS FOR OBSTRUCTIVE AIRWAY DISEASES
- R - RESPIRATORY SYSTEM
Active Ingredient:MONTELUKAST SODIUM
Active Ingredient UNII:U1O3J18SFL
Drugbank ID:DB00471
PubChem Compound:5281040
CTD ID:C093875
PharmGKB:PA450546
CAS Number:158966-92-8
Dosage Form(s):tablet, chewable
Route(s) Of Administrator:oral
Daily Dose:
- 10.0 mg/day R03DC03
Chemical Structure: 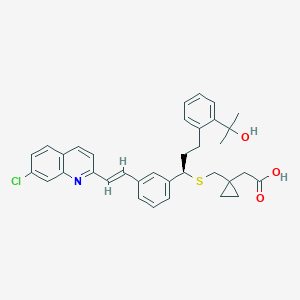

SMILE Code:
OC(=O)CC1(CC1)CS[C@H](CCC1=CC=CC=C1C(O)(C)C)C1=CC=CC(\C=C\C2=NC3=C(C=CC(Cl)=C3)C=C2)=C1
OC(=O)CC1(CC1)CS[C@H](CCC1=CC=CC=C1C(O)(C)C)C1=CC=CC(\C=C\C2=NC3=C(C=CC(Cl)=C3)C=C2)=C1
Reference
1: Drug interactions with cisapride: clinical implications.
[Michalets E L,Williams C R]Clin Pharmacokinet,2000 Jul;39(1):49-75. PMID: 10926350
Disclaimer:
The content of this database of QT prolongation is intended for educational and scientific research purposes only. It is not intended as a substitute for professional medical advice, diagnosis or treatment.
Any mention of commercial products is for clarification and not intended as endorsement.