Search for drugs:
Typing the drug name to query
ATAZANAVIR
DIR Classification
Classification:Moderate-DIQT concern
Severity Score:3.0
Description in Drug Labeling: View Full Labeling: SPL in DailyMed | PDF
- WARNINGS AND PRECAUTIONS
- Cardiac Conduction Abnormalities
- Atazanavir has been shown to prolong the PR interval of the electrocardiogram in some patients. In healthy volunteers and in patients, abnormalities in atrioventricular (AV) conduction were asymptomatic and generally limited to first-degree AV block. There have been reports of second-degree AV block and other conduction abnormalities [see Adverse Reactions ( 6.2) and Overdosage ( 10) ]. In clinical trials that included electrocardiograms, asymptomatic first-degree AV block was observed in 5.9% of atazanavir-treated patients (n=920), 5.2% of lopinavir/ritonavir-treated patients (n=252), 10.4% of nelfinavir-treated patients (n=48), and 3.0% of efavirenz-treated patients (n=329). In Study AI424-045, asymptomatic first-degree AV block was observed in 5% (6/118) of atazanavir/ritonavir-treated patients and 5% (6/116) of lopinavir/ritonavir-treated patients who had on-study electrocardiogram measurements. Because of limited clinical experience in patients with preexisting conduction system disease (eg, marked first-degree AV block or second- or third-degree AV block). ECG monitoring should be considered in these patients. [See Clinical Pharmacology ( 12.2) .]
- DRUG INTERACTIONS
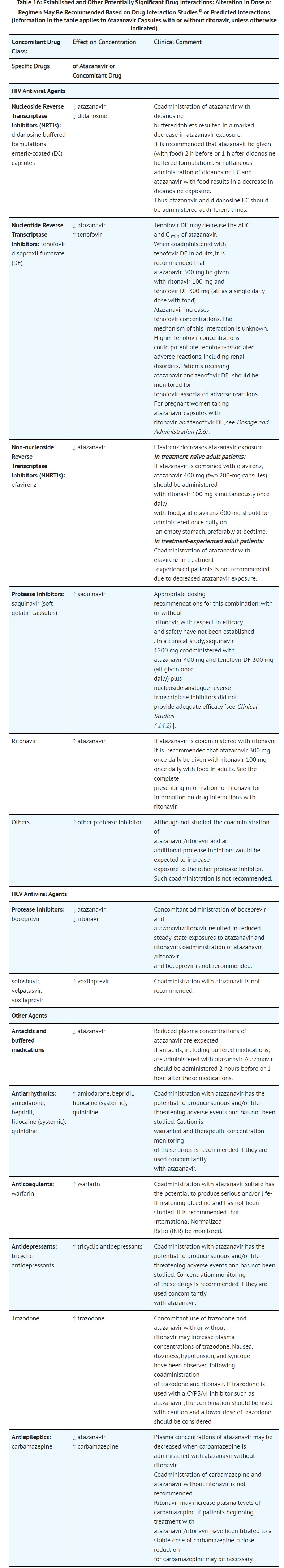
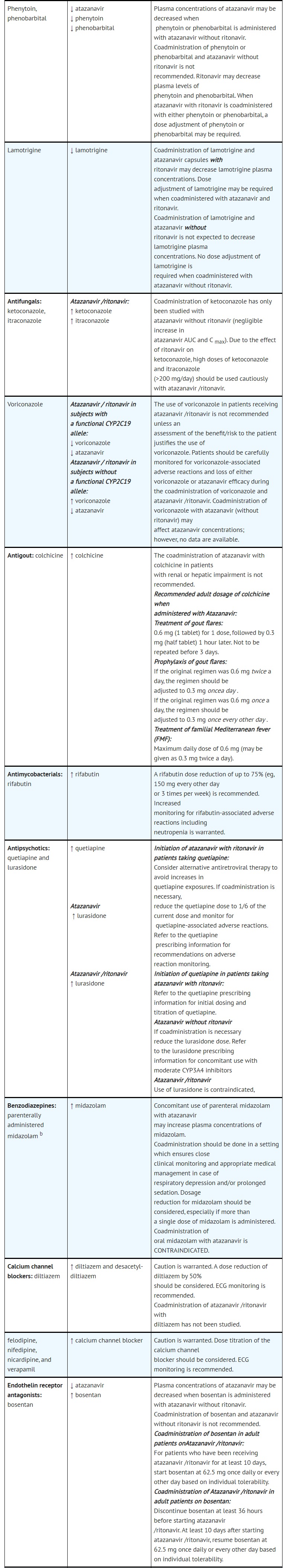
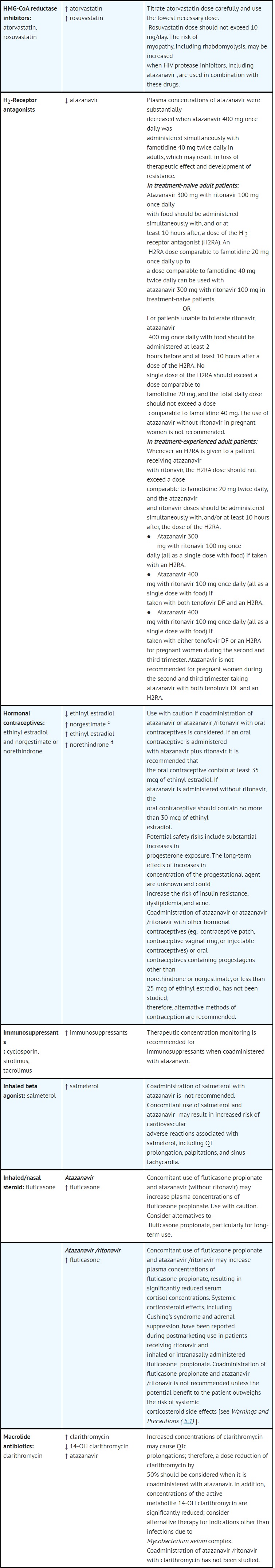
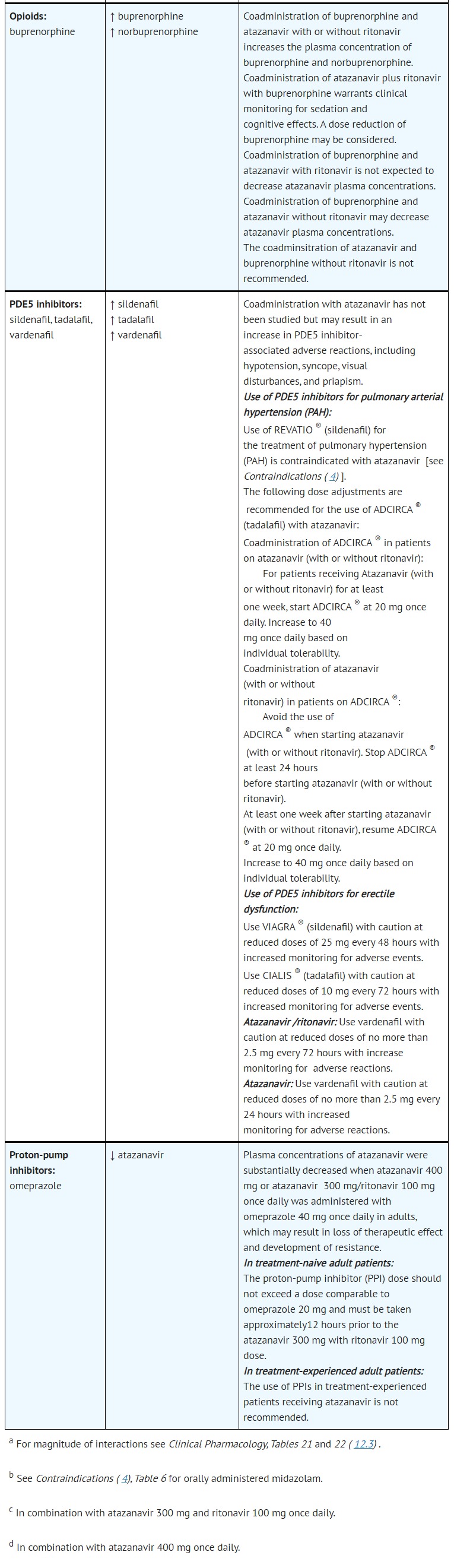
- ADVERSE REACTIONS
- Postmarketing Experience
- Cardiovascular System: second-degree AV block, third-degree AV block, left bundle branch block, QTc prolongation [see Warnings and Precautions ( 5.1) ]
- CLINICAL PHARMACOLOGY
- Pharmacodynamics
- Cardiac Electrophysiology
- Concentration- and dose-dependent prolongation of the PR interval in the electrocardiogram has been observed in healthy volunteers receiving atazanavir. In a placebo-controlled study (AI424-076), the mean (±SD) maximum change in PR interval from the predose value was 24 (±15) msec following oral dosing with 400 mg of atazanavir (n=65) compared to 13 (±11) msec following dosing with placebo (n=67). The PR interval prolongations in this study were asymptomatic. There is limited information on the potential for a pharmacodynamic interaction in humans between atazanavir and other drugs that prolong the PR interval of the electrocardiogram. [See Warnings and Precautions ( 5.1) .]
- Electrocardiographic effects of atazanavir were determined in a clinical pharmacology study of 72 healthy subjects. Oral doses of 400 mg (maximum recommended dosage) and 800 mg (twice the maximum recommended dosage) were compared with placebo; there was no concentration-dependent effect of atazanavir on the QTc interval (using Fridericia's correction). In 1793 HIV-infected patients receiving antiretroviral regimens, QTc prolongation was comparable in the atazanavir and comparator regimens. No atazanavir-treated healthy subject or HIV-infected patient in clinical trials had a QTc interval >500 msec. [See Warnings and Precautions ( 5.1) .]
Postmarketing Surveillance
Contingency Table:
Current Drug
Other Drugs
QT Prolongation
27
24065
Other ADRs
9847
38371740
Odds Ratio = 4.373
Drug Property Information
ATC Code(s):
- J05AE08 - atazanavir
- J05AE0 -
- J05AE - Protease inhibitors
- J05A - DIRECT ACTING ANTIVIRALS
- J05 - ANTIVIRALS FOR SYSTEMIC USE
- J - ANTIINFECTIVES FOR SYSTEMIC USE
Active Ingredient:ATAZANAVIR SULFATE
Active Ingredient UNII:4MT4VIE29P
Drugbank ID:DB01072
PubChem Compound:148192
CTD ID:D000069446
PharmGKB:PA10251
CAS Number:198904-31-3
Dosage Form(s):capsule, gelatin coated
Route(s) Of Administrator:oral
Daily Dose:
- 300.0 mg/day J05AE08
Chemical Structure: 

SMILE Code:
COC(=O)N[C@H](C(=O)N[C@@H](CC1=CC=CC=C1)[C@@H](O)CN(CC1=CC=C(C=C1)C1=CC=CC=N1)NC(=O)[C@@H](NC(=O)OC)C(C)(C)C)C(C)(C)C
COC(=O)N[C@H](C(=O)N[C@@H](CC1=CC=CC=C1)[C@@H](O)CN(CC1=CC=C(C=C1)C1=CC=CC=N1)NC(=O)[C@@H](NC(=O)OC)C(C)(C)C)C(C)(C)C
Reference
1: Effect of COVID-19 medications on corrected QT interval and induction of torsade de pointes: Results of a multicenter national survey.
[Haghjoo Majid,Golipra Reza,Kheirkhah Jalal,Golabchi Allahyar,Shahabi Javad,Oni-Heris Saeed,Sami Ramin,Tajmirriahi Marzieh,Saravi Mehrdad,Khatami Mozhdeh,Varnasseri Mehran,Kiarsi Mohammadreza,Hejazi Seyed Fakhreddin,Yousefzadeh Rahaghi Mojtaba,Taherkhani Maryam,Ashraf Haleh,Keshmiri Mohammad Sadegh,Akbarzadeh Mohammad Ali,Bozorgi Ali,Mottaghizadeh Fateme,Hedayat Behnam,Heidarali Mona,Hajhossein Talasaz Azita]Int J Clin Pract,2021 Mar 24;e14182. PMID: 33759318
2: Emerging and experimental treatments for COVID-19 and drug interactions with psychotropic agents.
[Bishara Delia,Kalafatis Chris,Taylor David]Ther Adv Psychopharmacol,2020 Jun 22;10:2045125320935306. PMID: 32612804
3: Monomorphic ventricular tachycardia due to protease inhibitor intoxication by atazanavir.
[Kim Byunghyun,Kim Kyung Su]Clin Exp Emerg Med,2018 Jun;5(2):131-134. PMID: 29706057
4: Drug-induced Inhibition and Trafficking Disruption of ion Channels: Pathogenesis of QT Abnormalities and Drug-induced Fatal Arrhythmias.
[Cubeddu Luigi X]Curr Cardiol Rev,2016;12(2):141-54. PMID: 26926294
5: Atazanavir induced first degree atrioventricular block and ventricular tachycardia: a case report.
[Santimaleeworagun Wichai,Pattharachayakul Suthipom,Chusri Sarunyou,Chayagul Pantip]J Med Assoc Thai,2013 Apr;96(4):501-3. PMID: 23691707
Disclaimer:
The content of this database of QT prolongation is intended for educational and scientific research purposes only. It is not intended as a substitute for professional medical advice, diagnosis or treatment.
Any mention of commercial products is for clarification and not intended as endorsement.