Search for drugs:
Typing the drug name to query
TIOTROPIUM BROMIDE
DIR Classification
Classification:Ambiguous
Severity Score:1.0
Description in Drug Labeling: View Full Labeling: SPL in DailyMed | PDF
- CLINICAL PHARMACOLOGY
- Pharmacodynamics
- Cardiac Electrophysiology
- In a multicenter, randomized, double-blind trial using tiotropium dry powder for inhalation that enrolled 198 patients with COPD, the number of subjects with changes from baseline-corrected QT interval of 30 to 60 msec was higher in the SPIRIVA HANDIHALER group as compared with placebo. This difference was apparent using both the Bazett (QTcB) [20 (20%) patients vs. 12 (12%) patients] and Fredericia (QTcF) [16 (16%) patients vs. 1 (1%) patient] corrections of QT for heart rate. No patients in either group had either QTcB or QTcF of >500 msec. Other clinical studies with SPIRIVA HANDIHALER did not detect an effect of the drug on QTc intervals.
- The effect of tiotropium dry powder for inhalation on QT interval was also evaluated in a randomized, placebo- and positive-controlled crossover study in 53 healthy volunteers. Subjects received tiotropium dry powder for inhalation 18 mcg, 54 mcg (3 times the recommended dose), or placebo for 12 days. ECG assessments were performed at baseline and throughout the dosing interval following the first and last dose of study medication. Relative to placebo, the maximum mean change from baseline in study-specific QTc interval was 3.2 msec and 0.8 msec for tiotropium dry powder for inhalation 18 mcg and 54 mcg, respectively. No subject showed a new onset of QTc >500 msec or QTc changes from baseline of ≥0 msec.
Postmarketing Surveillance
Contingency Table:
Current Drug
Other Drugs
QT Prolongation
9
24083
Other ADRs
130471
38251116
Odds Ratio = 0.11
Drug Property Information
ATC Code(s):
- R03BB04 - tiotropium bromide
- R03BB - Anticholinergics
- R03B - "OTHER DRUGS FOR OBSTRUCTIVE AIRWAY DISEASES, INHALANTS"
- R03 - DRUGS FOR OBSTRUCTIVE AIRWAY DISEASES
- R - RESPIRATORY SYSTEM
Active Ingredient:TIOTROPIUM BROMIDE MONOHYDRATE
Active Ingredient UNII:L64SXO195N
Drugbank ID:DB01409
PubChem Compound:5487427
CTD ID: D000069447
PharmGKB:PA164769056
CAS Number:186691-13-4
Dosage Form(s):capsule
Route(s) Of Administrator:oral; respiratory (inhalation)
Daily Dose:
- 0.01 mg/day R03BB04
Chemical Structure: 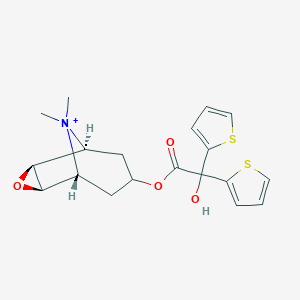

SMILE Code:
[H][C@]12O[C@@]1([H])[C@]1([H])C[C@@]([H])(C[C@@]2([H])[N+]1(C)C)OC(=O)C(O)(C1=CC=CS1)C1=CC=CS1
[H][C@]12O[C@@]1([H])[C@]1([H])C[C@@]([H])(C[C@@]2([H])[N+]1(C)C)OC(=O)C(O)(C1=CC=CS1)C1=CC=CS1
Reference
1: Absence of electrocardiographic findings and improved function with once-daily tiotropium in patients with chronic obstructive pulmonary disease.
[Covelli Henry,Bhattacharya Sudipta,Cassino Cara,Conoscenti Craig,Kesten Steven]Pharmacotherapy,2005 Dec;25(12):1708-18. PMID: 16305289
Disclaimer:
The content of this database of QT prolongation is intended for educational and scientific research purposes only. It is not intended as a substitute for professional medical advice, diagnosis or treatment.
Any mention of commercial products is for clarification and not intended as endorsement.