Search for drugs:
Typing the drug name to query
TELITHROMYCIN
DIR Classification
Classification:Most-DIQT concern
Severity Score:4.0
Description in Drug Labeling: View Full Labeling: SPL in DailyMed | PDF
- WARNINGS
- QTc prolongation
- Telithromycin has the potential to prolong the QTc interval of the electrocardiogram in some patients. QTc prolongation may lead to an increased risk for ventricular arrhythmias, including torsades de pointes. Thus, telithromycin should be avoided in patients with congenital prolongation of the QTc interval, and in patients with ongoing proarrhythmic conditions such as uncorrected hypokalemia or hypomagnesemia, clinically significant bradycardia, and in patients receiving Class IA (e.g., quinidine and procainamide) or Class III (e.g., dofetilide) antiarrhythmic agents.
- Cases of torsades de pointes have been reported post-marketing with KETEK. In clinical trials, no cardiovascular morbidity or mortality attributable to QTc prolongation occurred with telithromycin treatment in 4780 patients in clinical trials, including 204 patients having a prolonged QTc at baseline.
- PRECAUTIONS
- Information for patients
- KETEK has the potential to produce changes in the electrocardiogram (QTc interval prolongation) and that they should report any fainting occurring during drug treatment.
- KETEK should be avoided in patients receiving Class 1A (e.g., quinidine, procainamide) or Class III (e.g., dofetilide) antiarrhythmic agents.
- to inform their physician of any personal or family history of QTc prolongation or proarrhythmic conditions such as uncorrected hypokalemia, or clinically significant bradycardia.
- CLINICAL PHARMACOLOGY
- Drug-drug interactions
- CYP 3A4 substrates
- Cisapride
- Steady-state peak plasma concentrations of cisapride (an agent with the potential to increase QT interval) were increased by 95% when co-administered with repeated doses of telithromycin, resulting in significant increases in QTc. (See CONTRAINDICATIONS)
- MEDICATION GUIDE
- Before taking KETEK, tell your doctor if you:
- have liver problems
- have a heart problem called "QTc prolongation" or have a family history of QTc prolongation
- have other heart problems
- are pregnant or plan to become pregnant. It is not known if KETEK will harm your unborn baby. Talk to your doctor if you are pregnant or plan to become pregnant.
- ANIMAL PHARMACOLOGY
- Pharmacology/toxicology studies showed an effect both in prolonging QTc interval in dogs in vivo and in vitro action potential duration (APD) in rabbit Purkinje fibers. These effects were observed at concentrations of free drug at least 8.8 (in dogs) times those circulating in clinical use. In vitro electrophysiological studies (hERG assays) suggested an inhibition of the rapid activating component of the delayed rectifier potassium current (IKr) as an underlying mechanism.
Postmarketing Surveillance
Contingency Table:
Current Drug
Other Drugs
QT Prolongation
16
24076
Other ADRs
11124
38370463
Odds Ratio = 2.293
Drug Property Information
ATC Code(s):
- J01FA15 - telithromycin
- J01FA - Macrolides
- J01F - "MACROLIDES, LINCOSAMIDES AND STREPTOGRAMINS"
- J01 - ANTIBACTERIALS FOR SYSTEMIC USE
- J - ANTIINFECTIVES FOR SYSTEMIC USE
Active Ingredient:telithromycin
Active Ingredient UNII:KI8H7H19WL
Drugbank ID:DB00976
PubChem Compound:3002190
CTD ID:C106791
PharmGKB:PA10202
CAS Number:191114-48-4
Dosage Form(s):tablet, film coated
Route(s) Of Administrator:oral
Daily Dose:
- 800.0 mg/day J01FA15
Chemical Structure: 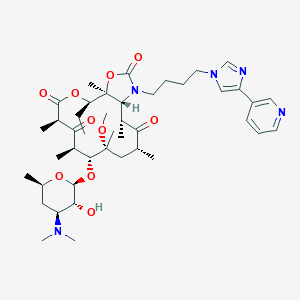

SMILE Code:
[H][C@@]12[C@@H](C)C(=O)[C@H](C)C[C@@](C)(OC)[C@H](O[C@@H]3O[C@H](C)C[C@@H]([C@H]3O)N(C)C)[C@@H](C)C(=O)[C@@H](C)C(=O)O[C@H](CC)[C@@]1(C)OC(=O)N2CCCCN1C=NC(=C1)C1=CC=CN=C1
[H][C@@]12[C@@H](C)C(=O)[C@H](C)C[C@@](C)(OC)[C@H](O[C@@H]3O[C@H](C)C[C@@H]([C@H]3O)N(C)C)[C@@H](C)C(=O)[C@@H](C)C(=O)O[C@H](CC)[C@@]1(C)OC(=O)N2CCCCN1C=NC(=C1)C1=CC=CN=C1
Reference
1: Telithromycin: visual disorders.
Prescrire Int,2010 Apr;19(106):71. PMID: 20568490
2: Ketolides: pharmacological profile and rational positioning in the treatment of respiratory tract infections.
[Van Bambeke Françoise,Harms Joerg M,Van Laethem Yves,Tulkens Paul M]Expert Opin Pharmacother,2008 Feb;9(2):267-83. PMID: 18201149
3: In-vitro experimental models for the risk assessment of antibiotic-induced QT prolongation.
[Lu Hua Rong,Vlaminckx Eddy,Van de Water Andre,Rohrbacher Jutta,Hermans An,Gallacher David J]Eur J Pharmacol,2007 Dec 22;577(1-3):222-32. PMID: 18074444
4: Telithromycin: QT prolongation.
Prescrire Int,2007 Apr;16(88):71. PMID: 17465034
5: In-vitro experimental models for the risk assessment of antibiotic-induced QT prolongation.
[Lu Hua Rong,Vlaminckx Eddy,Van de Water Andre,Rohrbacher Jutta,Hermans An,Gallacher David J]Eur J Pharmacol,2006 Dec 28;553(1-3):229-39. PMID: 17054943
6: Differentiation of arrhythmia risk of the antibacterials moxifloxacin, erythromycin, and telithromycin based on analysis of monophasic action potential duration alternans and cardiac instability.
[Wisialowski Todd,Crimin Kimberly,Engtrakul Juntyma,O'Donnell John,Fermini Bernard,Fossa Anthony A]J Pharmacol Exp Ther,2006 Jul;318(1):352-9. PMID: 16614168
Disclaimer:
The content of this database of QT prolongation is intended for educational and scientific research purposes only. It is not intended as a substitute for professional medical advice, diagnosis or treatment.
Any mention of commercial products is for clarification and not intended as endorsement.