Search for drugs:
Typing the drug name to query
HYDROCODONE BITARTRATE
DIR Classification
Classification:Moderate-DIQT concern
Severity Score:2.0
Description in Drug Labeling: View Full Labeling: SPL in DailyMed | PDF
- WARNINGS AND PRECAUTIONS
- QTc Interval Prolongation
- QTc prolongation has been observed with HYSINGLA ER following daily doses of 160 mg [see Clinical Pharmacology (12.2)]. This observation should be considered in making clinical decisions regarding patient monitoring when prescribing HYSINGLA ER in patients with congestive heart failure, bradyarrhythmias, electrolyte abnormalities, or who are taking medications that are known to prolong the QTc interval.
- HYSINGLA ER should be avoided in patients with congenital long QT syndrome. In patients who develop QTc prolongation, consider reducing the dose by 33 – 50%, or changing to an alternate analgesic.
- CLINICAL PHARMACOLOGY
- Pharmacodynamics
- Cardiac Electrophysiology
- QTc interval prolongation was studied in a double-blind, placebo- and positive-controlled 3-treatment parallel-group, dose-escalating study of HYSINGLA ER in 196 healthy subjects. QTc interval prolongation was observed following HYSINGLA ER 160 mg per day. The maximum mean (90% upper confidence bound) difference in the QTc interval between HYSINGLA ER and placebo (after baseline-correction) at steady state was 6 (9) milliseconds, 7 (10) milliseconds, and 10 (13) milliseconds at HYSINGLA ER doses of 80 mg, 120 mg and 160 mg respectively. For clinical implications of the prolonged QTc interval, see Warnings and Precautions (5.10).
- PATIENT COUNSELING INFORMATION
- QTc interval prolongation
- Inform patients that QT prolongation has been observed with HYSINGLA ER [see Clinical Pharmacology (12.2)]. HYSINGLA ER should be avoided in patients with congenital long QT syndrome. Instruct patients with a history of congestive heart failure or bradyarrhythmias, and patients at risk for electrolyte abnormalities or who are taking other medications known to prolong the QT interval, that periodic monitoring of electrocardiograms and electrolytes may be necessary during therapy with HYSINGLA ER [see Warnings and Precautions (5.10)].
Postmarketing Surveillance
Contingency Table:
Current Drug
Other Drugs
QT Prolongation
5
24087
Other ADRs
11539
38370048
Odds Ratio = 0.691
Drug Property Information
ATC Code(s):
- R05DA03 - hydrocodone bitartrate
- R05DA - Opium alkaloids and derivatives
- R05D - "COUGH SUPPRESSANTS, EXCL. COMBINATIONS WITH EXPECTORANTS"
- R05 - COUGH AND COLD PREPARATIONS
- R - RESPIRATORY SYSTEM
Active Ingredient:HYDROCODONE BITARTRATE
Active Ingredient UNII:NO70W886KK
Drugbank ID:DB00956
PubChem Compound:5284569
CTD ID:D006853
PharmGKB:PA449900
CAS Number:125-29-1
Dosage Form(s):tablet, extended release
Route(s) Of Administrator:oral
Daily Dose:
- 15.0 mg/day R05DA03
Chemical Structure: 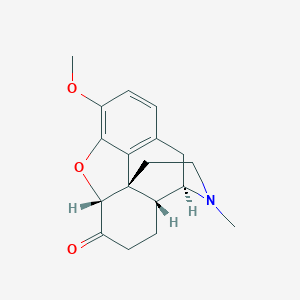

SMILE Code:
[H][C@@]12OC3=C(OC)C=CC4=C3[C@@]11CCN(C)[C@]([H])(C4)[C@]1([H])CCC2=O
[H][C@@]12OC3=C(OC)C=CC4=C3[C@@]11CCN(C)[C@]([H])(C4)[C@]1([H])CCC2=O
Reference
N/A
Disclaimer:
The content of this database of QT prolongation is intended for educational and scientific research purposes only. It is not intended as a substitute for professional medical advice, diagnosis or treatment.
Any mention of commercial products is for clarification and not intended as endorsement.