Search for drugs:
Typing the drug name to query
GILTERITINIB
DIR Classification
Classification:Most-DIQT concern
Severity Score:4.0
Description in Drug Labeling: View Full Labeling: SPL in DailyMed | PDF
- WARNINGS AND PRECAUTIONS
- Prolonged QT Interval
- XOSPATA has been associated with prolonged cardiac ventricular repolarization (QT interval). Of the 317 patients with a post-baseline QTc measurement on treatment with XOSPATA in the clinical trial, 1% were found to have a QTc interval greater than 500 msec and 7% of patients had an increase from baseline QTc greater than 60 msec. Perform electrocardiogram (ECG) prior to initiation of treatment with gilteritinib, on days 8 and 15 of cycle 1, and prior to the start of the next two subsequent cycles. Interrupt and reduce XOSPATA dosage in patients who have a QTcF >500 msec [see Dosage and Administration (2.3), Adverse Reactions (6.1) and Clinical Pharmacology (12.2)].
- Hypokalemia or hypomagnesemia may increase the QT prolongation risk. Correct hypokalemia or hypomagnesemia prior to and during XOSPATA administration.
- DOSAGE AND ADMINISTRATION
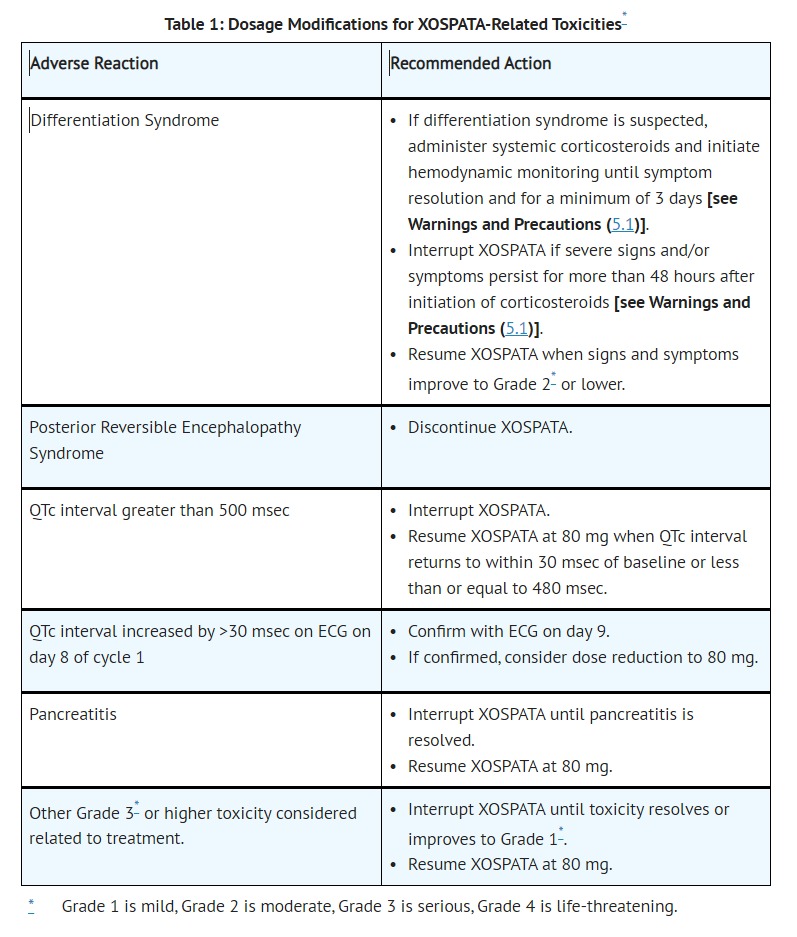
- ADVERSE REACTIONS
- The following clinically significant adverse reactions are described elsewhere in the labeling:
- Prolonged QT interval [see Warnings and Precautions (5.3)]
- [Clinical Trials Experience]
- Other clinically significant adverse reactions occurring in ≤10% of patients included: electrocardiogram QT prolonged (9%), hypersensitivity* (8%), pancreatitis* (5%), cardiac failure* (4%), pericardial effusion (4%), acute febrile neutrophilic dermatosis (3%), differentiation syndrome (3%), pericarditis/myocarditis* (2%), large intestine perforation (1%), and posterior reversible encephalopathy syndrome (1%).
- CLINICAL PHARMACOLOGY
- Pharmacodynamics
- Cardiac Electrophysiology
- The effect of XOSPATA 120 mg once a day on the QTc interval has been evaluated in patients, which showed an absence of large mean increases (i.e., 20 msec) in the QTc interval.
- Of 317 patients with a post-baseline QTc measurement on treatment with gilteritinib at 120 mg in clinical trials, 4 patients (1.3%) experienced a QTcF >500 msec. Additionally, across all doses 2.3% of patients with relapse/refractory AML had a maximum post-baseline QTcF interval >500 msec [see Warnings and Precautions (5.3)].
- PATIENT COUNSELING INFORMATION
- Prolonged QT Interval
- Advise patients to consult their healthcare provider immediately if they feel faint, lose consciousness, or have signs or symptoms suggestive of arrhythmia. Advise patients with a history of hypokalemia or hypomagnesemia of the importance of monitoring their electrolytes [see Warnings and Precautions (5.3)].
- MEDICATION GUIDE
- XOSPATA may cause serious side effects, including:
- Changes in the electrical activity of your heart called QTc prolongation. QTc prolongation can cause irregular heartbeats that can be life-threatening. Your healthcare provider will check the electrical activity of your heart with a test called an electrocardiogram (ECG) before you start taking XOSPATA and during your treatment with XOSPATA. Tell your healthcare provider right away if you feel dizzy, lightheaded, or faint. The risk of QT prolongation is higher in people with low blood magnesium or low blood potassium levels. Your healthcare provider will do blood tests to check your potassium and magnesium levels before and during your treatment with XOSPATA.
Postmarketing Surveillance
Contingency Table:
Current Drug
Other Drugs
QT Prolongation
26
24066
Other ADRs
3069
38378518
Odds Ratio = 13.511
Drug Property Information
ATC Code(s):
- L01EX13 - gilteritinib
- L01EX1 -
- L01EX -
- L01E -
- L01 - ANTINEOPLASTIC AGENTS
- L - ANTINEOPLASTIC AND IMMUNOMODULATING AGENTS
Active Ingredient:GILTERITINIB FUMARATE
Active Ingredient UNII:5RZZ0Z1GJT
Drugbank ID:DB12141
PubChem Compound:49803313
CTD ID:C000609080
CAS Number:1254053-43-4
Dosage Form(s):tablet
Route(s) Of Administrator:oral
Daily Dose:
Chemical Structure: 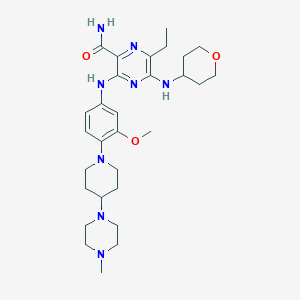

SMILE Code:
CCC1=C(NC2CCOCC2)N=C(NC2=CC=C(N3CCC(CC3)N3CCN(C)CC3)C(OC)=C2)C(=N1)C(N)=O
CCC1=C(NC2CCOCC2)N=C(NC2=CC=C(N3CCC(CC3)N3CCN(C)CC3)C(OC)=C2)C(=N1)C(N)=O
Reference
1: {'i': 'FLT3', '#text': 'FDA Approval Summary: Gilteritinib for Relapsed or Refractory Acute Myeloid Leukemia with a Mutation.'}
[Pulte E Dianne,Norsworthy Kelly J,Wang Yaping,Xu Qing,Qosa Hisham,Gudi Ramadevi,Przepiorka Donna,Fu Wentao,Okusanya Olanrewaju O,Goldberg Kirsten B,De Claro R Angelo,Farrell Ann T,Pazdur Richard]Clin Cancer Res,2021 Feb 25. PMID: 33632926
2: {'i': 'FLT3', '#text': 'Gilteritinib use in the treatment of relapsed or refractory acute myeloid leukemia with a mutation.'}
[Ballesta-López Octavio,Solana-Altabella Antonio,Megías-Vericat Juan Eduardo,Martínez-Cuadrón David,Montesinos Pau]Future Oncol,2021 Jan;17(2):215-227. PMID: 32975130
3: Drug-drug interactions of newly approved small molecule inhibitors for acute myeloid leukemia.
[Megías-Vericat Juan Eduardo,Solana-Altabella Antonio,Ballesta-López Octavio,Martínez-Cuadrón David,Montesinos Pau]Ann Hematol,2020 Sep;99(9):1989-2007. PMID: 32683457
Disclaimer:
The content of this database of QT prolongation is intended for educational and scientific research purposes only. It is not intended as a substitute for professional medical advice, diagnosis or treatment.
Any mention of commercial products is for clarification and not intended as endorsement.