Search for drugs:
Typing the drug name to query
ALOGLIPTIN
DIR Classification
Classification:Ambiguous
Severity Score:1.0
Description in Drug Labeling: View Full Labeling: SPL in DailyMed | PDF
- CLINICAL PHARMACOLOGY
- Pharmacodynamics
- Cardiac Electrophysiology
- In a randomized, placebo-controlled, four-arm, parallel-group study, 257 subjects were administered either alogliptin 50 mg, alogliptin 400 mg, moxifloxacin 400 mg or placebo once daily for a total of seven days. No increase in corrected QT (QTc) was observed with either dose of alogliptin. At the 400 mg dose, peak alogliptin plasma concentrations were 19 fold higher than the peak concentrations following the maximum recommended clinical dose of 25 mg.
Postmarketing Surveillance
Contingency Table:
Current Drug
Other Drugs
QT Prolongation
0
24092
Other ADRs
2499
38379088
Odds Ratio = 0.0
Drug Property Information
ATC Code(s):
- A10BH04 - alogliptin
- A10BH0 -
- A10BH - Dipeptidyl peptidase 4 (DPP-4) inhibitors
- A10B - "BLOOD GLUCOSE LOWERING DRUGS, EXCL. INSULINS"
- A10 - DRUGS USED IN DIABETES
- A - ALIMENTARY TRACT AND METABOLISM
- A10BD13 - alogliptin
- A10BD - Combinations of oral blood glucose lowering drugs
- A10B - "BLOOD GLUCOSE LOWERING DRUGS, EXCL. INSULINS"
- A10 - DRUGS USED IN DIABETES
- A - ALIMENTARY TRACT AND METABOLISM
- A10BD09 - alogliptin
- A10BD - Combinations of oral blood glucose lowering drugs
- A10B - "BLOOD GLUCOSE LOWERING DRUGS, EXCL. INSULINS"
- A10 - DRUGS USED IN DIABETES
- A - ALIMENTARY TRACT AND METABOLISM
Active Ingredient:Alogliptin Benzoate
Active Ingredient UNII:EEN99869SC
Drugbank ID:DB06203
PubChem Compound:11450633
CTD ID: C520853
CAS Number:850649-61-5
Dosage Form(s):tablet, film coated
Route(s) Of Administrator:oral
Daily Dose:
- 25.0 mg/day A10BH04
Chemical Structure: 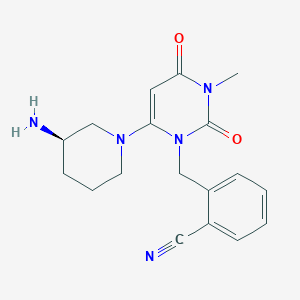

SMILE Code:
CN1C(=O)C=C(N2CCC[C@@H](N)C2)N(CC2=C(C=CC=C2)C#N)C1=O
CN1C(=O)C=C(N2CCC[C@@H](N)C2)N(CC2=C(C=CC=C2)C#N)C1=O
Reference
N/A
Disclaimer:
The content of this database of QT prolongation is intended for educational and scientific research purposes only. It is not intended as a substitute for professional medical advice, diagnosis or treatment.
Any mention of commercial products is for clarification and not intended as endorsement.