Search for drugs:
Typing the drug name to query
VENETOCLAX
DIR Classification
Classification:Ambiguous
Severity Score:1.0
Description in Drug Labeling: View Full Labeling: SPL in DailyMed | PDF
- CLINICAL PHARMACOLOGY
- Pharmacokinetics
- Cardiac Electrophysiology
- The effect of multiple doses of VENCLEXTA up to 1200 mg once daily (2 times the maximum approved recommended dosage) on the QTc interval was evaluated in an open-label, single-arm trial in 176 patients with previously treated hematologic malignancies. VENCLEXTA had no large effect on QTc interval (i.e., >20 ms) and there was no relationship between venetoclax exposure and change in QTc interval.
Postmarketing Surveillance
Contingency Table:
Current Drug
Other Drugs
QT Prolongation
4
24088
Other ADRs
41406
38340181
Odds Ratio = 0.154
Drug Property Information
ATC Code(s):
- L01XX52 - venetoclax
- L01XX - Other antineoplastic agents
- L01X - OTHER ANTINEOPLASTIC AGENTS
- L01 - ANTINEOPLASTIC AGENTS
- L - ANTINEOPLASTIC AND IMMUNOMODULATING AGENTS
Active Ingredient:Venetoclax
Active Ingredient UNII:N54AIC43PW
Drugbank ID:DB11581
PubChem Compound:49846579
CTD ID: C579720
PharmGKB:PA166153473
CAS Number:1257044-40-8
Dosage Form(s):kit; tablet, film coated
Route(s) Of Administrator:oral
Daily Dose:
Chemical Structure: 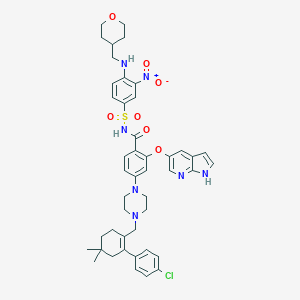

SMILE Code:
CC1(C)CCC(CN2CCN(CC2)C2=CC=C(C(=O)NS(=O)(=O)C3=CC=C(NCC4CCOCC4)C(=C3)[N+]([O-])=O)C(OC3=CN=C4NC=CC4=C3)=C2)=C(C1)C1=CC=C(Cl)C=C1
CC1(C)CCC(CN2CCN(CC2)C2=CC=C(C(=O)NS(=O)(=O)C3=CC=C(NCC4CCOCC4)C(=C3)[N+]([O-])=O)C(OC3=CN=C4NC=CC4=C3)=C2)=C(C1)C1=CC=C(Cl)C=C1
Reference
1: Drug-drug interactions of newly approved small molecule inhibitors for acute myeloid leukemia.
[Megías-Vericat Juan Eduardo,Solana-Altabella Antonio,Ballesta-López Octavio,Martínez-Cuadrón David,Montesinos Pau]Ann Hematol,2020 Sep;99(9):1989-2007. PMID: 32683457
2: Venetoclax does not prolong the QT interval in patients with hematological malignancies: an exposure-response analysis.
[Freise Kevin J,Dunbar Martin,Jones Aksana K,Hoffman David,Enschede Sari L Heitner,Wong Shekman,Salem Ahmed Hamed]Cancer Chemother Pharmacol,2016 Oct;78(4):847-53. PMID: 27586967
Disclaimer:
The content of this database of QT prolongation is intended for educational and scientific research purposes only. It is not intended as a substitute for professional medical advice, diagnosis or treatment.
Any mention of commercial products is for clarification and not intended as endorsement.