Search for drugs:
Typing the drug name to query
IOPAMIDOL
DIR Classification
Classification:Ambiguous
Severity Score:1.0
Description in Drug Labeling: View Full Labeling: SPL in DailyMed | PDF
- ADVERSE REACTIONS
- Following coronary and ventricular injections, certain electrocardiographic changes (increased QTc, increased R-R, T-wave amplitude) and certain hemodynamic changes (decreased systolic pressure) occurred less frequently with ISOVUE (lopamidol Injection) than with diatrizoate meglumine and diatrizoate sodium injection; increased LVEDP occurred less frequently after ventricular iopamidol injections.
Postmarketing Surveillance
Contingency Table:
Current Drug
Other Drugs
QT Prolongation
1
24091
Other ADRs
3984
38377603
Odds Ratio = 0.4
Drug Property Information
ATC Code(s):
- V08AB04 - iopamidol
- V08AB - "Watersoluble, nephrotropic, low osmolar X-ray contrast media"
- V08A - "X-RAY CONTRAST MEDIA, IODINATED"
- V08 - CONTRAST MEDIA
- V - VARIOUS
Active Ingredient:IOPAMIDOL
Active Ingredient UNII:JR13W81H44
Drugbank ID:DB08947
PubChem Compound:65492
CTD ID:D007479
CAS Number:60166-93-0
Dosage Form(s):injection, solution
Route(s) Of Administrator:intravascular
Daily Dose:
Chemical Structure: 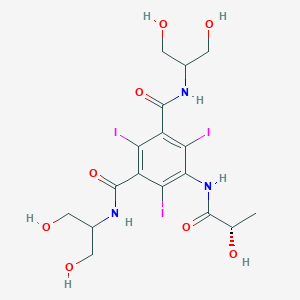

SMILE Code:
C[C@H](O)C(=O)NC1=C(I)C(C(=O)NC(CO)CO)=C(I)C(C(=O)NC(CO)CO)=C1I
C[C@H](O)C(=O)NC1=C(I)C(C(=O)NC(CO)CO)=C(I)C(C(=O)NC(CO)CO)=C1I
Reference
N/A
Disclaimer:
The content of this database of QT prolongation is intended for educational and scientific research purposes only. It is not intended as a substitute for professional medical advice, diagnosis or treatment.
Any mention of commercial products is for clarification and not intended as endorsement.