Search for drugs:
Typing the drug name to query
DEXMEDETOMIDINE HCL
DIR Classification
Classification:Ambiguous
Severity Score:1.0
Description in Drug Labeling: View Full Labeling: SPL in DailyMed | PDF
- ADVERSE REACTIONS
- Postmarketing Experience
- Adverse Reactions Experienced During Post-approval Use of Dexmedetomidine hydrochloride Investigations
- Alanine aminotransferase increased, aspartate aminotransferase increased, blood alkaline phosphatase increased, blood urea increased, electrocardiogram T wave inversion, gammaglutamyltransferase increased, electrocardiogram QT prolonged
Postmarketing Surveillance
Contingency Table:
Current Drug
Other Drugs
QT Prolongation
18
24074
Other ADRs
3568
38378019
Odds Ratio = 8.043
Drug Property Information
ATC Code(s):
- N05CM18 - dexmedetomidine hcl
- N05CM - Other hypnotics and sedatives
- N05C - HYPNOTICS AND SEDATIVES
- N05 - PSYCHOLEPTICS
- N - NERVOUS SYSTEM
Active Ingredient:DEXMEDETOMIDINE HYDROCHLORIDE
Active Ingredient UNII:1018WH7F9I
Drugbank ID:DB00633
PubChem Compound:5311068
CTD ID:D020927
PharmGKB:PA449256
CAS Number:113775-47-6
Dosage Form(s):injection
Route(s) Of Administrator:intravenous
Daily Dose:
- 1.0 mg/day N05CM18
Chemical Structure: 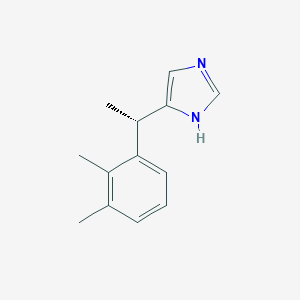

SMILE Code:
C[C@H](C1=CNC=N1)C1=C(C)C(C)=CC=C1
C[C@H](C1=CNC=N1)C1=C(C)C(C)=CC=C1
Reference
N/A
Disclaimer:
The content of this database of QT prolongation is intended for educational and scientific research purposes only. It is not intended as a substitute for professional medical advice, diagnosis or treatment.
Any mention of commercial products is for clarification and not intended as endorsement.