Search for drugs:
Typing the drug name to query
QUINIDINE GLUCONATE
DIR Classification
Classification:Most-DIQT concern
Severity Score:4.0
Description in Drug Labeling: View Full Labeling: SPL in DailyMed | PDF
- WARNINGS
- Proarrhythmic effects
- Like many other drugs (including all other Class Ia antiarrhythmics), quinidine prolongs the QT c interval, and this can lead to torsades de pointes, a life-threatening ventricular arrhythmia (see OVERDOSAGE). The risk of torsades is increased by bradycardia, hypokalemia, hypomagnesemia or high serum levels of quinidine, but it may appear in the absence of any of these risk factors. The best predictor of this arrhythmia appears to be the length of QT c interval, and quinidine should be used with extreme care in patients who have preexisting long-QT syndromes, who have histories of torsades de pointes of any cause, or who have previously responded to quinidine (or other drugs that prolong ventricular repolarization) with marked lengthening of the QT c interval. Estimation of the incidence of torsades in patients with therapeutic levels of quinidine is not possible from the available data.
- Other ventricular arrhythmias that have been reported with quinidine include frequent extrasystoles, ventricular tachycardia, ventricular flutter, and ventricular fibrillation.
- OVERDOSAGE
- Arrhythmias
- Serum quinidine levels can be conveniently assayed and monitored, but the electrocardiographic QT c interval is a better predictor of quinidine-induced ventricular arrhythmias.
- The necessary treatment of hemodynamically unstable polymorphic ventricular tachycardia (including torsades de pointes) is withdrawal of treatment with quinidine and either immediate cardioversion or, if a cardiac pacemaker is in place or immediately available, immediate overdrive pacing. After pacing or cardioversion, further management must be guided by the length of the QT c interval.
- Quinidine-associated ventricular tachyarrhythmias with normal underlying QT c intervals have not been adequately studied. Because of the theoretical possibility of QT-prolonging effects that might be additive to those of quinidine, other antiarrhythmics with Class I (disopyramide, procainamide) or Class III activities should (if possible) be avoided. Similarly, although the use of bretylium in quinidine overdose has not been reported, it is reasonable to expect that the α-blocking properties of bretylium might be additive to those of quinidine, resulting in problematic hypotension.
- If the post-cardioversion QT c interval is prolonged, then the pre-cardioversion polymorphic ventricular tachycardia was (by definition) torsades de pointes. In this case, lidocaine and bretylium are unlikely to be of value, and other Class I antiarrhythmics (disopyramide, procainamide) are likely to exacerbate the situation. Factors contributing to QT c prolongation (especially hypokalemia and hypomagnesemia) should be sought out and (if possible) aggressively corrected. Prevention of recurrent torsades may require sustained overdrive pacing or the cautious administration of isoproterenol (30 to 150 ng/kg/min).
- DOSAGE AND ADMINISTRATION
- Conversion of atrial fibrillation/flutter to sinus rhythm
- Especially in patients with known structural heart disease or other risk factors for toxicity, initiation or dose-adjustment of treatment with quinidine gluconate should generally be performed in a setting where facilities and personnel for monitoring and resuscitation are continuously available. Patients with symptomatic atrial fibrillation/flutter should be treated with quinidine gluconate only after ventricular rate control (e.g., with digitalis or β-blockers) has failed to provide satisfactory control of symptoms.
- Adequate trials have not identified an optimal regimen of quinidine gluconate for conversion of atrial fibrillation/flutter to sinus rhythm. In one reported regimen, the patient first receives two tablets (648 mg; 403 mg of quinidine base) of quinidine gluconate every eight hours. If this regimen has not resulted in conversion after 3 or 4 doses, then the dose is cautiously increased. If, at any point during administration, the QRS complex widens to 130% of its pre-treatment duration; the QT c interval widens to 130% of its pre-treatment duration and is then longer than 500 ms; P waves disappear; or the patient develops significant tachycardia, symptomatic bradycardia, or hypotension, then quinidine gluconate is discontinued, and other means of conversion (e.g., direct-current cardioversion) are considered.
- In another regimen sometimes used, the patient receives one tablet (324 mg; 202 mg of quinidine base) every eight hours for two days; then two tablets every twelve hours for two days; and finally two tablets every eight hours for up to four days. The four-day stretch may come at one of the lower doses if, in the judgment of the physician, the lower dose is the highest one that will be tolerated. The criteria for discontinuation of treatment with quinidine gluconate are the same as in the other regimen.
- [Reduction in the frequency of relapse into atrial fibrillation/flutter]
- In a patient with a history of frequent symptomatic episodes of atrial fibrillation/flutter, the goal of therapy with quinidine gluconate should be an increase in the average time between episodes. In most patients, the tachyarrhythmia will recur during therapy with quinidine gluconate, and a single recurrence should not be interpreted as therapeutic failure.
- Especially in patients with known structural heart disease or other risk factors for toxicity, initiation or dose-adjustment of treatment with quinidine gluconate should generally be performed in a setting where facilities and personnel for monitoring and resuscitation are continuously available. Monitoring should be continued for two or three days after initiation of the regimen on which the patient will be discharged.
- Therapy with quinidine gluconate should be begun with one tablet (324 mg; 202 mg of quinidine base) every eight or twelve hours. If this regimen is well tolerated, if the serum quinidine level is still well within the laboratory's therapeutic range, and if the average time between arrhythmic episodes has not been satisfactorily increased, then the dose may be cautiously raised. The total daily dosage should be reduced if the QRS complex widens to 130% of its pre-treatment duration; the QT c interval widens to 130% of its pre-treatment duration and is then longer than 500 ms; P waves disappear; or the patient develops significant tachycardia, symptomatic bradycardia, or hypotension.
- [Suppression of life-threatening ventricular arrhythmias]
- Dosing regimens for the use of quinidine gluconate in suppressing life-threatening ventricular arrhythmias have not been adequately studied. Described regimens have generally been similar to the regimen described just above for the prophylaxis of symptomatic atrial fibrillation/flutter. Where possible, therapy should be guided by the results of programmed electrical stimulation and/or Holter monitoring with exercise.
- CLINICAL PHARMACOLOGY
- Pharmacokinetics and Metabolism
- As measured by antiarrhythmic effects on animals, by QT c prolongation in human volunteers, or by various in vitro techniques, 3HQ has at least half the antiarrhythmic activity of the parent compound, so it may be responsible for a substantial fraction of the effect of quinidine gluconate in chronic use.
- [Mechanisms of action]
- Like other antiarrhythmic drugs with Class Ia activity, quinidine prolongs the QT interval in a dose-related fashion. This may lead to increased ventricular automaticity and polymorphic ventricular tachycardias, including torsades de pointes (see WARNINGS).
- CLINICAL EFFECTS
- Non-life-threatening ventricular arrhythmias: In studies of patients with a variety of ventricular arrhythmias (mainly frequent ventricular premature beats and non-sustained ventricular tachycardia, quinidine (total n=502) has been compared with flecainide (n=141), mexiletine (n=246), propafenone (n=53), and tocainide (n=67). In each of these studies, the mortality in the quinidine group was numerically greater than the mortality in the comparator group. When the studies were combined in a meta-analysis, quinidine was associated with a statistically significant threefold relative risk of death.
- At therapeutic doses, quinidine's only consistent effect upon the surface electrocardiogram is an increase in the QT interval. This prolongation can be monitored as a guide to safety, and it may provide better guidance than serum drug levels (see WARNINGS).
Postmarketing Surveillance
Contingency Table:
Current Drug
Other Drugs
QT Prolongation
39
24053
Other ADRs
3908
38377679
Odds Ratio = 15.923
Drug Property Information
ATC Code(s):
- C01BA01 - quinidine gluconate
- C01BA - "Antiarrhythmics, class Ia"
- C01B - "ANTIARRHYTHMICS, CLASS I AND III"
- C01 - CARDIAC THERAPY
- C - CARDIOVASCULAR SYSTEM
Active Ingredient:QUINIDINE GLUCONATE
Active Ingredient UNII:R6875N380F
Drugbank ID:DB00908
PubChem Compound:441074
CTD ID:D011802
PharmGKB:PA451209
CAS Number:56-54-2
Dosage Form(s):tablet, extended release
Route(s) Of Administrator:oral
Daily Dose:
- 1200.0 mg/day C01BA01
Chemical Structure: 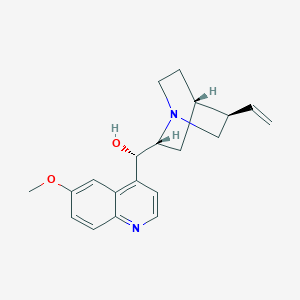

SMILE Code:
[H][C@@]12CCN(C[C@@H]1C=C)[C@]([H])(C2)[C@@H](O)C1=C2C=C(OC)C=CC2=NC=C1
[H][C@@]12CCN(C[C@@H]1C=C)[C@]([H])(C2)[C@@H](O)C1=C2C=C(OC)C=CC2=NC=C1
Reference
N/A
Disclaimer:
The content of this database of QT prolongation is intended for educational and scientific research purposes only. It is not intended as a substitute for professional medical advice, diagnosis or treatment.
Any mention of commercial products is for clarification and not intended as endorsement.