Search for drugs:
Typing the drug name to query
AMISULPRIDE
DIR Classification
Classification:Moderate-DIQT concern
Severity Score:3.0
Description in Drug Labeling: View Full Labeling: SPL in DailyMed | PDF
- WARNINGS AND PRECAUTIONS
- QT Prolongation
- BARHEMSYS causes dose- and concentration-dependent prolongation of the QT interval [see CLINICAL PHARMACOLOGY (12.2)]. The recommended dosage is 5 or 10 mg as a single intravenous dose infused over 1 to 2 minutes [see DOSAGE AND ADMINISTRATION (2.1)].
- Avoid BARHEMSYS in patients with congenital long QT syndrome and in patients taking droperidol.
- Electrocardiogram (ECG) monitoring is recommended in patients with pre-existing arrhythmias/cardiac conduction disorders; electrolyte abnormalities (e.g., hypokalemia or hypomagnesemia); congestive heart failure; and in patients taking other medicinal products (e.g., ondansetron) or with other medical conditions known to prolong the QT interval [see DRUG INTERACTIONS (7.2)].
- DRUG INTERACTIONS
- Drugs Prolonging the QT Interval
- BARHEMSYS causes dose- and concentration-dependent QT prolongation [see CLINICAL PHARMACOLOGY (12.2)]. To avoid potential additive effects, avoid use of BARHEMSYS in patients taking droperidol. ECG monitoring is recommended in patients taking other drugs known to prolong the QT interval (e.g., ondansetron) [see WARNINGS AND PRECAUTIONS (5.1)].
- OVERDOSAGE
- Doses of oral amisulpride (BARHEMSYS is not approved for oral dosing) above 1200 mg/day have been associated with adverse reactions related to dopamine-2 (D2) antagonism, in particular:
- cardiovascular adverse reactions (e.g., prolongation of the QT interval, torsades de pointes, bradycardia and hypotension) [see WARNINGS AND PRECAUTIONS (5.1)].
- neuropsychiatric adverse reactions (e.g., sedation, coma, seizures, and dystonic and extrapyramidal reactions).
- ADVERSE REACTIONS
- Postmarketing Experience
- The following adverse reactions have been identified during postapproval chronic oral use of amisulpride outside of the United States (BARHEMSYS is not approved for oral dosing or chronic use). Because these reactions are reported voluntarily from a population of uncertain size, it is not always possible to reliably estimate their frequency or establish a causal relationship to drug exposure.
- Blood and lymphatic system disorders: agranulocytosis
- Cardiac disorders: bradycardia, torsades de pointes, ventricular tachycardia, prolonged QT by electrocardiogram
- General disorders: neuroleptic malignant syndrome
- Immune system disorders: angioedema, hypersensitivity, urticaria
- Hepatic disorders: increased hepatic enzymes
- Nervous system disorders: agitation, anxiety, dystonia, extrapyramidal disorder, seizure
- Psychiatric disorders: confusional state, insomnia, somnolence
- Vascular disorders: hypotension
- CLINICAL PHARMACOLOGY
- Pharmacodynamics
- Cardiac Electrophysiology
- In 40 healthy Caucasian and Japanese subjects, the maximum mean difference (95% upper confidence bound) in QTcF from placebo after baseline-correction (ΔΔQTcF) was 5.0 (7.1) milliseconds after a 2-minute intravenous infusion of 5 mg BARHEMSYS and 23.4 (25.5) milliseconds after an 8-minute intravenous infusion of 40 mg BARHEMSYS [see WARNINGS AND PRECAUTIONS (5.1)].
- A significant exposure-response relationship was identified between amisulpride concentration and ΔΔQTcF. Using this exposure-response relationship, 10 mg BARHEMSYS infused intravenously over 1 min has a maximal predicted (95% upper prediction interval) ΔΔQTcF of 13.4 (15.1) milliseconds.
- The recommended infusion rate is 1 to 2 minutes for 5 mg or 10 mg of BARHEMSYS [see DOSAGE AND ADMINISTRATION (2.1)].
- PATIENT COUNSELING INFORMATION
- QT Prolongation
- Instruct patients to contact their healthcare provider immediately if they perceive a change in their heart rate, if they feel lightheaded, or if they have a syncopal episode [see WARNINGS AND PRECAUTIONS (5.1)].
- [Drug Interactions]
- Advise patients to report to their healthcare provider if they are taking drugs which prolong the QT interval [see WARNINGS AND PRECAUTIONS (5.1)].
Postmarketing Surveillance
Contingency Table:
Current Drug
Other Drugs
QT Prolongation
7
24085
Other ADRs
362
38381225
Odds Ratio = 30.815
Drug Property Information
ATC Code(s):
- N05AL05 - amisulpride
- N05AL0 -
- N05AL - Benzamides
- N05A - ANTIPSYCHOTICS
- N05 - PSYCHOLEPTICS
- N - NERVOUS SYSTEM
Active Ingredient:AMISULPRIDE
Active Ingredient UNII:8110R61I4U
Drugbank ID:DB06288
PubChem Compound:2159
CTD ID:D000077582
PharmGKB:PA162565877
CAS Number:71675-85-9
Dosage Form(s):injection, solution
Route(s) Of Administrator:intravenous
Daily Dose:
- 400.0 mg/day N05AL05
Chemical Structure: 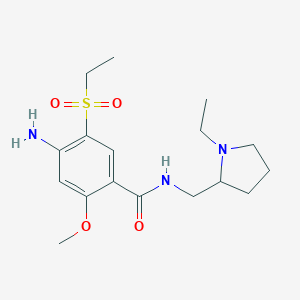

SMILE Code:
CCN1CCCC1CNC(=O)C1=CC(=C(N)C=C1OC)S(=O)(=O)CC
CCN1CCCC1CNC(=O)C1=CC(=C(N)C=C1OC)S(=O)(=O)CC
Reference
1: Amisulpride: A New Drug for Management of Postoperative Nausea and Vomiting.
[Haber Stacy L,Graybill April,Minasian Ani]Ann Pharmacother,2021 Jan 8;1060028020987012. PMID: 33412897
2: Arrhythmias related to antipsychotics and antidepressants: an analysis of the summaries of product characteristics of original products approved in Germany.
[Elsayed Mohamed,Abdel-Kahaar Emaad,Gahr Maximilian,Connemann Bernhard J,Denkinger Michael,Schönfeldt-Lecuona Carlos]Eur J Clin Pharmacol,2021 May;77(5):767-775. PMID: 33230596
3: Drugs for preventing postoperative nausea and vomiting in adults after general anaesthesia: a network meta-analysis.
[Weibel Stephanie,Rücker Gerta,Eberhart Leopold Hj,Pace Nathan L,Hartl Hannah M,Jordan Olivia L,Mayer Debora,Riemer Manuel,Schaefer Maximilian S,Raj Diana,Backhaus Insa,Helf Antonia,Schlesinger Tobias,Kienbaum Peter,Kranke Peter]Cochrane Database Syst Rev,2020 Oct 19;10:CD012859. PMID: 33075160
4: Risk of Prolonged Corrected QT Interval With Amisulpride Therapy for Renal Function Management in Patients With Schizophrenia.
[Chen Binbin,Wang Chen,Xu Xiangzhen,Lyu Haiyan,Ma Chunling,Cheng Gang]J Clin Psychopharmacol,Sep/Oct 2020;40(5):482-486. PMID: 32826486
5: QT interval prolongation noted in one percent of 2553 Asian patients with schizophrenia: Findings from the REAP-AP survey.
[Park Seon-Cheol,Lee Bong Ju,Park Jae Hong,Kawasaki Hiroaki,Avasthi Ajit,Grover Sandeep,Tanra Andi J,Lin Shih-Ku,Javed Afzal,Tan Chay Hoon,Sartorius Norman,Shinfuku Naotaka,Park Yong Chon]Kaohsiung J Med Sci,2020 Dec;36(12):1030-1037. PMID: 32772489
6: Intravenous Amisulpride Does Not Meaningfully Prolong the QTc Interval at Doses Effective for the Management of Postoperative Nausea and Vomiting.
[Fox Gabriel M,Albayaty Muna,Walker Joanna L,Xue Hongqi,Darpo Borje]Anesth Analg,2021 Jan;132(1):150-159. PMID: 31913911
7: Comparative efficacy and tolerability of 32 oral antipsychotics for the acute treatment of adults with multi-episode schizophrenia: a systematic review and network meta-analysis.
[Huhn Maximilian,Nikolakopoulou Adriani,Schneider-Thoma Johannes,Krause Marc,Samara Myrto,Peter Natalie,Arndt Thomas,Bäckers Lio,Rothe Philipp,Cipriani Andrea,Davis John,Salanti Georgia,Leucht Stefan]Lancet,2019 Sep 14;394(10202):939-951. PMID: 31303314
8: Maximizing response to first-line antipsychotics in schizophrenia: a review focused on finding from meta-analysis.
[Smith Robert C,Leucht Stefan,Davis John M]Psychopharmacology (Berl),2019 Feb;236(2):545-559. PMID: 30506237
9: Diurnal Profile of the QTc Interval Following Moxifloxacin Administration.
[Täubel Jörg,Ferber Georg,Fernandes Sara,Camm A John]J Clin Pharmacol,2019 Jan;59(1):35-44. PMID: 30040135
10: QTc prolongation in short-term treatment of schizophrenia patients: effects of different antipsychotics and genetic factors.
[Spellmann Ilja,Reinhard Matthias A,Veverka Diana,Zill Peter,Obermeier Michael,Dehning Sandra,Schennach Rebecca,Müller Norbert,Möller Hans-Jürgen,Riedel Michael,Musil Richard]Eur Arch Psychiatry Clin Neurosci,2018 Jun;268(4):383-390. PMID: 29429138
11: Intravenous Amisulpride for the Prevention of Postoperative Nausea and Vomiting: Two Concurrent, Randomized, Double-blind, Placebo-controlled Trials.
[Gan Tong J,Kranke Peter,Minkowitz Harold S,Bergese Sergio D,Motsch Johann,Eberhart Leopold,Leiman David G,Melson Timothy I,Chassard Dominique,Kovac Anthony L,Candiotti Keith A,Fox Gabriel,Diemunsch Pierre]Anesthesiology,2017 Feb;126(2):268-275. PMID: 27902493
12: The Contribution of National Spontaneous Reporting Systems to Detect Signals of Torsadogenicity: Issues Emerging from the ARITMO Project.
[Raschi Emanuel,Poluzzi Elisabetta,Salvo Francesco,Koci Ariola,Suling Marc,Antoniazzi Stefania,Perina Luisella,Hazell Lorna,Moretti Ugo,Sturkenboom Miriam,Garbe Edeltraut,Pariente Antoine,De Ponti Fabrizio]Drug Saf,2016 Jan;39(1):59-68. PMID: 26446144
13: The Half RR Rule: A Poor Rule of Thumb and Not a Risk Assessment Tool for QT Interval Prolongation.
[Berling Ingrid,Isbister Geoffrey K]Acad Emerg Med,2015 Oct;22(10):1139-44. PMID: 26375169
14: Prolonged QT Risk Assessment in Antipsychotic Overdose Using the QT Nomogram.
[Berling Ingrid,Isbister Geoffrey K]Ann Emerg Med,2015 Aug;66(2):154-64. PMID: 25639523
15: Torsadogenic risk of antipsychotics: combining adverse event reports with drug utilization data across Europe.
[Raschi Emanuel,Poluzzi Elisabetta,Godman Brian,Koci Ariola,Moretti Ugo,Kalaba Marija,Bennie Marion,Barbui Corrado,Wettermark Bjorn,Sturkenboom Miriam,De Ponti Fabrizio]PLoS One,2013 Nov 20;8(11):e81208. PMID: 24278396
16: Quetiapine versus other atypical antipsychotics for schizophrenia.
[Asmal Laila,Flegar Srnka J,Wang Jikun,Rummel-Kluge Christine,Komossa Katja,Leucht Stefan]Cochrane Database Syst Rev,2013 Nov 18;(11):CD006625. PMID: 24249315
17: Comparative efficacy and tolerability of 15 antipsychotic drugs in schizophrenia: a multiple-treatments meta-analysis.
[Leucht Stefan,Cipriani Andrea,Spineli Loukia,Mavridis Dimitris,Orey Deniz,Richter Franziska,Samara Myrto,Barbui Corrado,Engel Rolf R,Geddes John R,Kissling Werner,Stapf Marko Paul,Lässig Bettina,Salanti Georgia,Davis John M]Lancet,2013 Sep 14;382(9896):951-62. PMID: 23810019
18: QTc prolongation by psychotropic drugs and the risk of Torsade de Pointes.
[Wenzel-Seifert Katharina,Wittmann Markus,Haen Ekkehard]Dtsch Arztebl Int,2011 Oct;108(41):687-93. PMID: 22114630
19: Prediction of torsade de pointes from the QT interval: analysis of a case series of amisulpride overdoses.
[Joy J P,Coulter C V,Duffull S B,Isbister G K]Clin Pharmacol Ther,2011 Aug;90(2):243-5. PMID: 21716272
20: Amisulpride overdose is frequently associated with QT prolongation and torsades de pointes.
[Isbister Geoffrey K,Balit Corrine R,Macleod Dawson,Duffull Stephen B]J Clin Psychopharmacol,2010 Aug;30(4):391-5. PMID: 20531221
21: Quetiapine versus other atypical antipsychotics for schizophrenia.
[Komossa Katja,Rummel-Kluge Christine,Schmid Franziska,Hunger Heike,Schwarz Sandra,Srisurapanont Manit,Kissling Werner,Leucht Stefan]Cochrane Database Syst Rev,2010 Jan 20;(1):CD006625. PMID: 20091600
22: Aripiprazole versus other atypical antipsychotics for schizophrenia.
[Komossa Katja,Rummel-Kluge Christine,Schmid Franziska,Hunger Heike,Schwarz Sandra,El-Sayeh Hany George G,Kissling Werner,Leucht Stefan]Cochrane Database Syst Rev,2009 Oct 7;(4):CD006569. PMID: 19821375
23: Sertindole versus other atypical antipsychotics for schizophrenia.
[Komossa Katja,Rummel-Kluge Christine,Hunger Heike,Schwarz Sandra,Schmidt Franziska,Lewis Ruth,Kissling Werner,Leucht Stefan]Cochrane Database Syst Rev,2009 Apr 15;(2):CD006752. PMID: 19370652
24: Torsade de pointes associated with low-dose amisulpride: a case report.
[Chung A K K,Chua S E]J Psychopharmacol,2010 Mar;24(3):433-5. PMID: 18957479
25: Fatality due to amisulpride toxicity: a case report.
[Lynch Matthew J,Woods Jessica,George Natalia,Gerostamoulos Dimitri]Med Sci Law,2008 Apr;48(2):173-7. PMID: 18533580
26: Sertindole: new drug. Another "atypical" neuroleptic; QT prolongation.
Prescrire Int,2007 Apr;16(88):59-62. PMID: 17458045
27: [Differences between men and women in side effects of second-generation antipsychotics].
[Aichhorn W,Whitworth A B,Weiss E M,Hinterhuber H,Marksteiner J]Nervenarzt,2007 Jan;78(1):45-52. PMID: 16874502
28: Amisulpride deliberate self-poisoning causing severe cardiac toxicity including QT prolongation and torsades de pointes.
[Isbister Geoffrey K,Murray Lindsay,John Sally,Hackett L Peter,Haider Tedo,O'Mullane Phebe,Gosselin Sophie,Daly Frank]Med J Aust,2006 Apr 3;184(7):354-6. PMID: 16584372
29: Antipsychotic drugs and QT prolongation.
[Stöllberger Claudia,Huber Johannes O,Finsterer Josef]Int Clin Psychopharmacol,2005 Sep;20(5):243-51. PMID: 16096514
30: Two cases of amisulpride overdose: a cause for prolonged QT syndrome.
[Ward David Ian]Emerg Med Australas,2005 Jun;17(3):274-6. PMID: 15953230
31: [QT prolongation and second-generation antipsychotics: overdose and therapeutic dosage].
[Trojak Benoît,Pinoit Jean-Michel,Disson-Dautriche Anne,Bonin Bernard,Giusselmann André]Therapie,Sep-Oct 2004;59(5):558-61. PMID: 15648310
32: [QT prolongation and torsade de pointes--tachycardia in therapy with maprotiline].
[Züchner Helmut]Dtsch Med Wochenschr,2002 May 3;127(18):983-4. PMID: 11987023
33: Asymptomatic bradycardia associated with amisulpride.
[Pedrosa Gil F,Grohmann R,Rüther E]Pharmacopsychiatry,2001 Nov;34(6):259-61. PMID: 11778148
34: [Acute poisoning by new psychotropic drugs].
[Harry P]Rev Prat,1997 Apr 1;47(7):731-5. PMID: 9183949
Disclaimer:
The content of this database of QT prolongation is intended for educational and scientific research purposes only. It is not intended as a substitute for professional medical advice, diagnosis or treatment.
Any mention of commercial products is for clarification and not intended as endorsement.