Search for drugs:
Typing the drug name to query
RUCAPARIB
DIR Classification
Classification:Ambiguous
Severity Score:1.0
Description in Drug Labeling: View Full Labeling: SPL in DailyMed | PDF
- ADVERSE REACTIONS
- Clinical Trials Experience
- Discontinuation due to adverse reactions occurred in 8% of patients receiving Rubraca. None of the adverse reactions leading to discontinuation of Rubraca (ECG QT prolonged, acute respiratory distress syndrome, anemia, balance disorder, cardiac failure, decreased appetite/fatigue/weight decreased, leukopenia/neutropenia, ALT/AST increased, and pneumonia) occurred in more than one patient (<1%).
- CLINICAL PHARMACOLOGY
- Cardiac Electrophysiology
- The effect of multiple doses of Rubraca on QTc interval was evaluated in an open-label single-arm study in 56 patients with solid tumors who were administered continuous doses of Rubraca ranging from 40 mg once daily (0.03 times the approved recommended dose) to 840 mg twice daily (1.4 times the approved recommended dose). A positive concentration-QTc relationship was observed. The mean QTcF increase from baseline (90% confidence interval [CI]) in population pharmacokinetics estimated 95th percentile Cmax (3019 ng/mL) at steady state of 600 mg rucaparib twice daily was 14.9 msec (11.1-18.7 msec).
Postmarketing Surveillance
Contingency Table:
Current Drug
Other Drugs
QT Prolongation
2
24090
Other ADRs
27628
38353959
Odds Ratio = 0.116
Drug Property Information
ATC Code(s):
- L01XK03 - rucaparib
- L01XK -
- L01X - OTHER ANTINEOPLASTIC AGENTS
- L01 - ANTINEOPLASTIC AGENTS
- L - ANTINEOPLASTIC AND IMMUNOMODULATING AGENTS
Active Ingredient:rucaparib camsylate
Active Ingredient UNII:41AX9SJ8KO
Drugbank ID:DB12332
PubChem Compound:9931954
CTD ID: C531549
PharmGKB:PA166163418
CAS Number:283173-50-2
Dosage Form(s):tablet, film coated
Route(s) Of Administrator:oral
Daily Dose:
Chemical Structure: 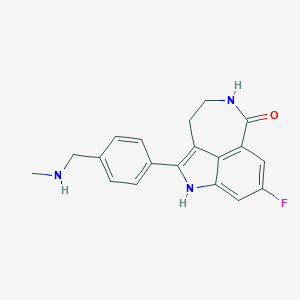

SMILE Code:
CNCC1=CC=C(C=C1)C1=C2CCNC(=O)C3=C2C(N1)=CC(F)=C3
CNCC1=CC=C(C=C1)C1=C2CCNC(=O)C3=C2C(N1)=CC(F)=C3
Reference
1: PARP inhibitor-induced torsades de pointes in long QT syndrome: a case report.
[Segan Louise,Beekman Ashley,Parfrey Shane,Perrin Mark]Eur Heart J Case Rep,2019 Dec 31;4(1):1-5. PMID: 32128485
Disclaimer:
The content of this database of QT prolongation is intended for educational and scientific research purposes only. It is not intended as a substitute for professional medical advice, diagnosis or treatment.
Any mention of commercial products is for clarification and not intended as endorsement.