Search for drugs:
Typing the drug name to query
TAMOXIFEN CITRATE
DIR Classification
Classification:Moderate-DIQT concern
Severity Score:2.0
Description in Drug Labeling: View Full Labeling: SPL in DailyMed | PDF
- OVERDOSAGE
- In the same study, prolongation of the QT interval on the electrocardiogram was noted when patients were given doses higher than 250 mg/m2 loading dose, followed by maintenance doses of 80 mg/m2 of tamoxifen citrate given twice a day. For a woman with a body surface area of 1.5 m2 the minimal loading dose and maintenance doses given at which neurological symptoms and QT changes occurred were at least 6 fold higher in respect to the maximum recommended dose.
Postmarketing Surveillance
Contingency Table:
Current Drug
Other Drugs
QT Prolongation
26
24066
Other ADRs
11200
38370387
Odds Ratio = 3.702
Drug Property Information
ATC Code(s):
- L02BA01 - tamoxifen citrate
- L02BA - Anti-estrogens
- L02B - HORMONE ANTAGONISTS AND RELATED AGENTS
- L02 - ENDOCRINE THERAPY
- L - ANTINEOPLASTIC AND IMMUNOMODULATING AGENTS
Active Ingredient:TAMOXIFEN CITRATE
Active Ingredient UNII:7FRV7310N6
Drugbank ID:DB00675
PubChem Compound:2733526
CTD ID:D013629
PharmGKB:PA451581
CAS Number:10540-29-1
Dosage Form(s):tablet, film coated
Route(s) Of Administrator:oral
Daily Dose:
- 20.0 mg/day L02BA01
Chemical Structure: 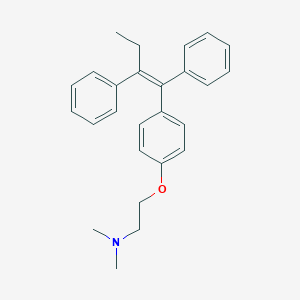

SMILE Code:
CC\C(=C(/C1=CC=CC=C1)C1=CC=C(OCCN(C)C)C=C1)C1=CC=CC=C1
CC\C(=C(/C1=CC=CC=C1)C1=CC=C(OCCN(C)C)C=C1)C1=CC=CC=C1
Reference
N/A
Disclaimer:
The content of this database of QT prolongation is intended for educational and scientific research purposes only. It is not intended as a substitute for professional medical advice, diagnosis or treatment.
Any mention of commercial products is for clarification and not intended as endorsement.