Search for drugs:
Typing the drug name to query
ROLAPITANT
DIR Classification
Classification:Most-DIQT concern
Severity Score:4.0
Description in Drug Labeling: View Full Labeling: SPL in DailyMed | PDF
- WARNINGS AND PRECAUTIONS
- Interaction with CYP2D6 Substrates
- Narrow Therapeutic Index Drugs (Thioridazine and Pimozide)
- VARUBI is contraindicated in patients taking CYP2D6 substrates with a narrow therapeutic index such as thioridazine and pimozide. Increased plasma concentrations of thioridazine and pimozide are associated with serious and/or life-threatening events of QT prolongation and Torsades de Pointes [see CONTRAINDICATIONS (4)].
- DRUG INTERACTIONS
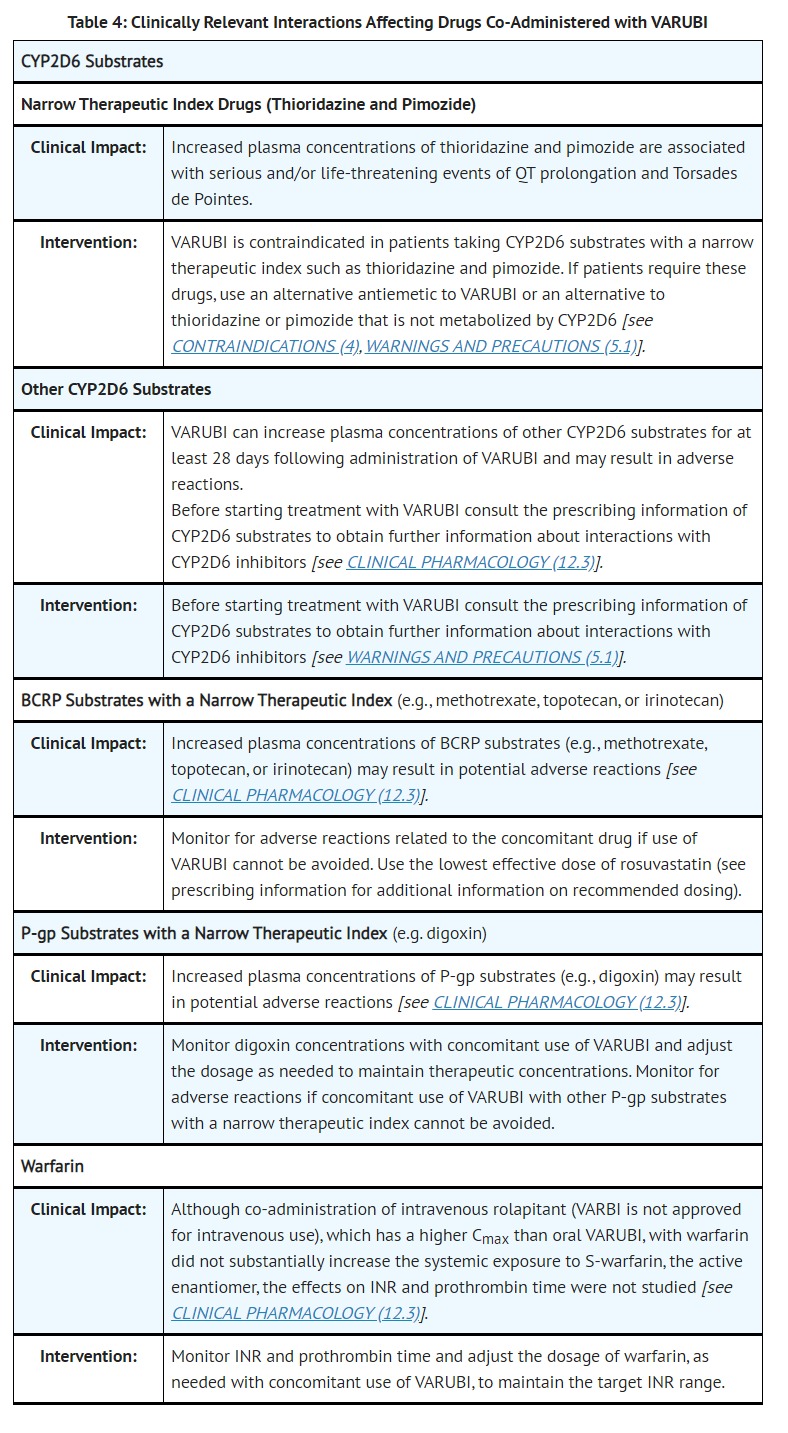
- CONTRAINDICATIONS
- VARUBI is contraindicated in patients taking CYP2D6 substrates with a narrow therapeutic index, such as thioridazine and pimozide. VARUBI can significantly increase the plasma concentrations of thioridazine and pimozide, which may result in QT prolongation and Torsades de Pointes [see WARNINGS AND PRECAUTIONS (5.1)].
- CLINICAL PHARMACOLOGY
- Pharmacodynamics
- Cardiac Electrophysiology
- At an oral dose of 720 mg (4 times the recommended oral dose), VARUBI does not prolong the QT interval to any clinically relevant extent.
Postmarketing Surveillance
Contingency Table:
Current Drug
Other Drugs
QT Prolongation
0
24092
Other ADRs
0
38381587
Odds Ratio = N/A
Drug Property Information
ATC Code(s):
- A04AD14 - rolapitant
- A04AD - Other antiemetics
- A04A - ANTIEMETICS AND ANTINAUSEANTS
- A04 - ANTIEMETICS AND ANTINAUSEANTS
- A - ALIMENTARY TRACT AND METABOLISM
Active Ingredient:ROLAPITANT HYDROCHLORIDE
Active Ingredient UNII:57O5S1QSAQ
Drugbank ID:DB09291
PubChem Compound:10311306
CTD ID:C578834
CAS Number:552292-08-7
Dosage Form(s):tablet
Route(s) Of Administrator:oral
Daily Dose:
- 180.0 mg/day A04AD14
Chemical Structure: 

SMILE Code:
C[C@@H](OC[C@]1(CC[C@]2(CCC(=O)N2)CN1)C1=CC=CC=C1)C1=CC(=CC(=C1)C(F)(F)F)C(F)(F)F
C[C@@H](OC[C@]1(CC[C@]2(CCC(=O)N2)CN1)C1=CC=CC=C1)C1=CC(=CC(=C1)C(F)(F)F)C(F)(F)F
Reference
1: Drugs for preventing postoperative nausea and vomiting in adults after general anaesthesia: a network meta-analysis.
[Weibel Stephanie,Rücker Gerta,Eberhart Leopold Hj,Pace Nathan L,Hartl Hannah M,Jordan Olivia L,Mayer Debora,Riemer Manuel,Schaefer Maximilian S,Raj Diana,Backhaus Insa,Helf Antonia,Schlesinger Tobias,Kienbaum Peter,Kranke Peter]Cochrane Database Syst Rev,2020 Oct 19;10:CD012859. PMID: 33075160
2: A Phase 1 Assessment of the QT Interval in Healthy Adults Following Exposure to Rolapitant, a Cancer Supportive Care Antiemetic.
[Wang Xiaodong,Zhang Zhi-Yi,Wang Jing,Kansra Vikram]Clin Pharmacol Drug Dev,2019 Jul;8(5):603-611. PMID: 30256537
Disclaimer:
The content of this database of QT prolongation is intended for educational and scientific research purposes only. It is not intended as a substitute for professional medical advice, diagnosis or treatment.
Any mention of commercial products is for clarification and not intended as endorsement.