Search for drugs:
Typing the drug name to query
AMBRISENTAN
DIR Classification
Classification:Ambiguous
Severity Score:1.0
Description in Drug Labeling: View Full Labeling: SPL in DailyMed | PDF
- CLINICAL PHARMACOLOGY
- Pharmacodynamics
- Cardiac Electrophysiology
- In a randomized, positive- and placebo-controlled, parallel-group study, healthy subjects received either ambrisentan 10 mg daily followed by a single dose of 40 mg, placebo followed by a single dose of moxifloxacin 400 mg, or placebo alone. Ambrisentan 10 mg daily had no significant effect on the QTc interval. The 40 mg dose of ambrisentan increased mean QTc at tmax by 5 ms with an upper 95% confidence limit of 9 ms. For patients receiving ambrisentan 5 mg to 10 mg daily and not taking metabolic inhibitors, no significant QT prolongation is expected.
Postmarketing Surveillance
Contingency Table:
Current Drug
Other Drugs
QT Prolongation
3
24089
Other ADRs
186882
38194705
Odds Ratio = 0.026
Drug Property Information
ATC Code(s):
- C02KX02 - ambrisentan
- C02KX0 -
- C02KX - Other antihypertensives
- C02K - OTHER ANTIHYPERTENSIVES
- C02 - ANTIHYPERTENSIVES
- C - CARDIOVASCULAR SYSTEM
- C02KX52 - ambrisentan
- C02KX - Other antihypertensives
- C02K - OTHER ANTIHYPERTENSIVES
- C02 - ANTIHYPERTENSIVES
- C - CARDIOVASCULAR SYSTEM
Active Ingredient:AMBRISENTAN
Active Ingredient UNII:HW6NV07QEC
Drugbank ID:DB06403
PubChem Compound:6918493
CTD ID:C467894
PharmGKB:PA165860521
CAS Number:177036-94-1
Dosage Form(s):tablet, film coated
Route(s) Of Administrator:oral
Daily Dose:
- 7.5 mg/day C02KX02
Chemical Structure: 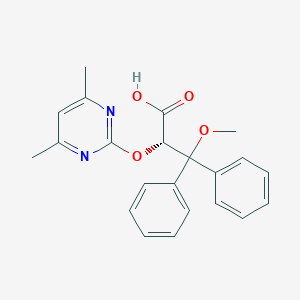

SMILE Code:
COC([C@H](OC1=NC(C)=CC(C)=N1)C(O)=O)(C1=CC=CC=C1)C1=CC=CC=C1
COC([C@H](OC1=NC(C)=CC(C)=N1)C(O)=O)(C1=CC=CC=C1)C1=CC=CC=C1
Reference
1: Evaluation system for arrhythmogenic potential of drugs using human-induced pluripotent stem cell-derived cardiomyocytes and gene expression analysis.
[Higa Arisa,Hoshi Hirotaka,Yanagisawa Yuka,Ito Emi,Morisawa Gaku,Imai Jun-Ichi,Watanabe Shinya,Takagi Motoki]J Toxicol Sci,2017;42(6):755-761. PMID: 29142174
Disclaimer:
The content of this database of QT prolongation is intended for educational and scientific research purposes only. It is not intended as a substitute for professional medical advice, diagnosis or treatment.
Any mention of commercial products is for clarification and not intended as endorsement.