Search for drugs:
Typing the drug name to query
TRANEXAMIC ACID
DIR Classification
Classification:Ambiguous
Severity Score:1.0
Description in Drug Labeling: View Full Labeling: SPL in DailyMed | PDF
- CLINICAL PHARMACOLOGY
- Pharmacodynamics
- Cardiac Electrophysiology
- The effect of tranexamic acid on QT interval was evaluated in a randomized, single-dose, 4-way crossover study in 48 healthy females aged 18 to 49 years. Subjects received (1) tranexamic acid 1300 mg (two 650 mg tablets), (2) tranexamic acid 3900 mg (six 650 mg tablets; three times the recommended single dose), (3) moxifloxacin 400 mg, and (4) placebo. There was no significant increase in the corrected QT interval at any time up to 24 hours after the administration of either dose of tranexamic acid. Moxifloxacin, the active control, was associated with a maximum 14.11 msec mean increase in corrected QT interval (moxifloxacin 鈥?placebo) at 3 hours after administration.
Postmarketing Surveillance
Contingency Table:
Current Drug
Other Drugs
QT Prolongation
0
24092
Other ADRs
3255
38378332
Odds Ratio = 0.0
Drug Property Information
ATC Code(s):
- B02AA02 - tranexamic acid
- B02AA0 -
- B02AA - Amino acids
- B02A - ANTIFIBRINOLYTICS
- B02 - ANTIHEMORRHAGICS
- B - BLOOD AND BLOOD FORMING ORGANS
Active Ingredient:Tranexamic Acid
Active Ingredient UNII:6T84R30KC1
Drugbank ID:DB00302
PubChem Compound:5526
CTD ID:D014148
PharmGKB:PA164750514
CAS Number:1197-18-8
Dosage Form(s):tablet
Route(s) Of Administrator:oral
Daily Dose:
- 2000.0 mg/day B02AA02
Chemical Structure: 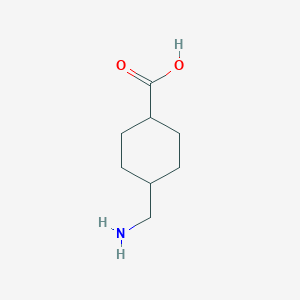

SMILE Code:
NC[C@H]1CC[C@@H](CC1)C(O)=O
NC[C@H]1CC[C@@H](CC1)C(O)=O
Reference
1: Treatment of Chronic Pulmonary Aspergillosis: Current Standards and Future Perspectives.
[Alastruey-Izquierdo Ana,Cadranel Jacques,Flick Holger,Godet Cendrine,Hennequin Christophe,Hoenigl Martin,Kosmidis Chris,Lange Christoph,Munteanu Oxana,Page Iain,Salzer Helmut J F,on behalf of CPAnet]Respiration,2018;96(2):159-170. PMID: 29982245
Disclaimer:
The content of this database of QT prolongation is intended for educational and scientific research purposes only. It is not intended as a substitute for professional medical advice, diagnosis or treatment.
Any mention of commercial products is for clarification and not intended as endorsement.