Search for drugs:
Typing the drug name to query
EFAVIRENZ
DIR Classification
Classification:Moderate-DIQT concern
Severity Score:3.0
Description in Drug Labeling: View Full Labeling: SPL in DailyMed | PDF
- WARNINGS AND PRECAUTIONS
- Drug Interactions
- Efavirenz plasma concentrations may be altered by substrates, inhibitors, or inducers of CYP3A. Likewise, efavirenz may alter plasma concentrations of drugs metabolized by CYP3A or CYP2B6. The most prominent effect of efavirenz at steady-state is induction of CYP3A and CYP2B6 [see DOSAGE AND ADMINISTRATION (2.2) and DRUG INTERACTIONS (7.1)].
- [QTc Prolongation]
- QTc prolongation has been observed with the use of efavirenz [see DRUG INTERACTIONS (7.3, 7.4) and CLINICAL PHARMACOLOGY (12.2)]. Consider alternatives to efavirenz when coadministered with a drug with a known risk of Torsade de Pointes or when administered to patients at higher risk of Torsade de Pointes.
- DRUG INTERACTIONS
- QT Prolonging Drugs
- There is limited information available on the potential for a pharmacodynamic interaction between efavirenz and drugs that prolong the QTc interval. QTc prolongation has been observed with the use of efavirenz [see CLINICAL PHARMACOLOGY (12.2)]. Consider alternatives to efavirenz when coadministered with a drug with a known risk of Torsade de Pointes.
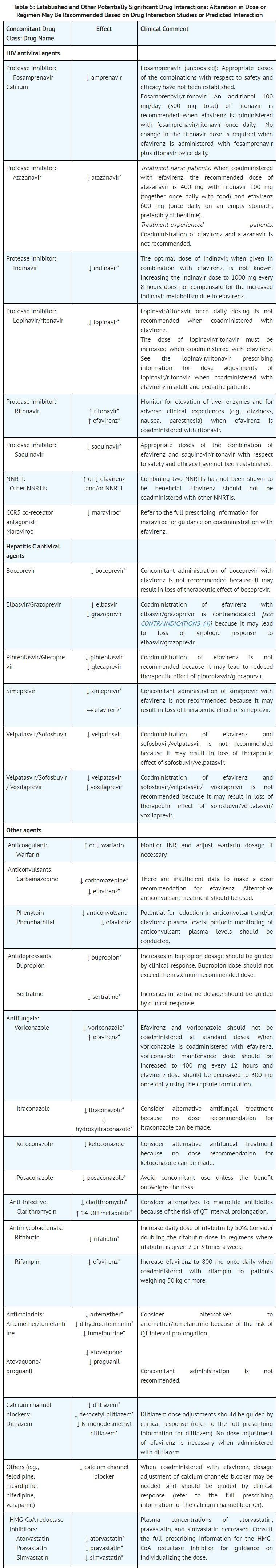
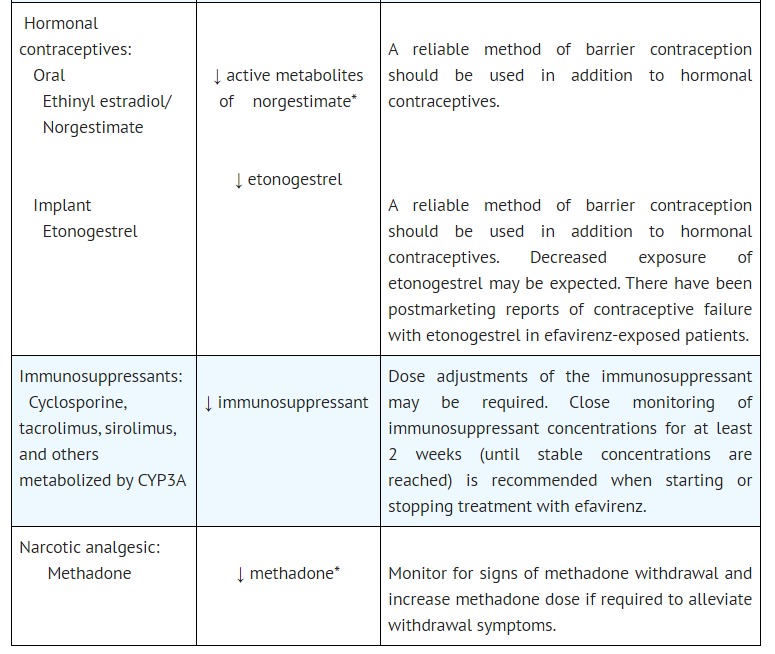
- CLINICAL PHARMACOLOGY
- Pharmacodynamics
- Cardiac Electrophysiology
- The effect of efavirenz on the QTc interval was evaluated in an open-label, positive and placebo controlled, fixed single sequence 3-period, 3-treatment crossover QT study in 58 healthy subjects enriched for CYP2B6 polymorphisms. The mean Cmax of efavirenz in subjects with CYP2B6 *6/*6 genotype following the administration of 600 mg daily dose for 14 days was 2.25-fold the mean Cmax observed in subjects with CYP2B6 *1/*1 genotype. A positive relationship between efavirenz concentration and QTc prolongation was observed. Based on the concentration-QTc relationship, the mean QTc prolongation and its upper bound 90% confidence interval are 8.7 ms and 11.3 ms in subjects with CYP2B6*6/*6 genotype following the administration of 600 mg daily dose for 14 days [see WARNINGS AND PRECAUTIONS (5.2)].
Postmarketing Surveillance
Contingency Table:
Current Drug
Other Drugs
QT Prolongation
8
24084
Other ADRs
34024
38347563
Odds Ratio = 0.375
Drug Property Information
ATC Code(s):
- J05AG03 - efavirenz
- J05AG - Non-nucleoside reverse transcriptase inhibitors
- J05A - DIRECT ACTING ANTIVIRALS
- J05 - ANTIVIRALS FOR SYSTEMIC USE
- J - ANTIINFECTIVES FOR SYSTEMIC USE
Active Ingredient:EFAVIRENZ
Active Ingredient UNII:JE6H2O27P8
Drugbank ID:DB00625
PubChem Compound:64139
CTD ID:C098320
PharmGKB:PA449441
CAS Number:154598-52-4
Dosage Form(s):capsule
Route(s) Of Administrator:oral
Daily Dose:
- 600.0 mg/day J05AG03
Chemical Structure: 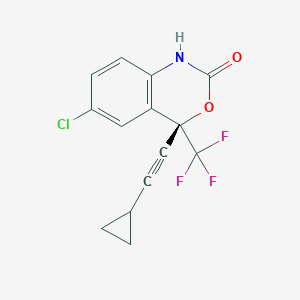

SMILE Code:
FC(F)(F)[C@]1(OC(=O)NC2=C1C=C(Cl)C=C2)C#CC1CC1
FC(F)(F)[C@]1(OC(=O)NC2=C1C=C(Cl)C=C2)C#CC1CC1
Reference
1: Predicting Optimal Dihydroartemisinin-Piperaquine Regimens to Prevent Malaria During Pregnancy for Human Immunodeficiency Virus-Infected Women Receiving Efavirenz.
[Wallender Erika,Vucicevic Katarina,Jagannathan Prasanna,Huang Liusheng,Natureeba Paul,Kakuru Abel,Muhindo Mary,Nakalembe Mirium,Havlir Diane,Kamya Moses,Aweeka Francesca,Dorsey Grant,Rosenthal Philip J,Savic Radojka M]J Infect Dis,2018 Mar 5;217(6):964-972. PMID: 29272443
2: Efavirenz Inhibits the Human Ether-A-Go-Go Related Current (hERG) and Induces QT Interval Prolongation in CYP2B6*6*6 Allele Carriers.
[Abdelhady Ahmed M,Shugg Tyler,Thong Nancy,Lu Jessica Bo Li,Kreutz Yvonne,Jaynes Heather A,Robarge Jason D,Tisdale James E,Desta Zeruesenay,Overholser Brian R]J Cardiovasc Electrophysiol,2016 Oct;27(10):1206-1213. PMID: 27333947
3: Potential interactions between HIV drugs, ritonavir and efavirenz and anticancer drug, nilotinib--a study in primary cultures of human hepatocytes that is applicable to HIV patients with cancer.
[Pillai Venkateswaran C,Parise Robert A,Christner Susan M,Rudek Michelle A,Beumer Jan H,Venkataramanan Raman]J Clin Pharmacol,2014 Nov;54(11):1272-9. PMID: 24846165
4: Rilpivirine: a new non-nucleoside reverse transcriptase inhibitor.
[Sharma Mamta,Saravolatz Louis D]J Antimicrob Chemother,2013 Feb;68(2):250-6. PMID: 23099850
5: Protease inhibitor-associated QT interval prolongation.
[Hunt Kimberley,Hughes Christine A,Hills-Nieminen Cara]Ann Pharmacother,2011 Dec;45(12):1544-50. PMID: 22128044
6: Role of antiretroviral treatment in prolonging QTc interval in HIV-positive patients.
[Chinello Pierangelo,Lisena Francesco P,Angeletti Claudio,Boumis Evangelo,Papetti Federica,Petrosillo Nicola]J Infect,2007 Jun;54(6):597-602. PMID: 17174400
7: Efavirenz-associated QT prolongation and Torsade de Pointes arrhythmia.
[Castillo Ricardo,Pedalino Ronald P,El-Sherif Nabil,Turitto Gioia]Ann Pharmacother,2002 Jun;36(6):1006-8. PMID: 12022902
Disclaimer:
The content of this database of QT prolongation is intended for educational and scientific research purposes only. It is not intended as a substitute for professional medical advice, diagnosis or treatment.
Any mention of commercial products is for clarification and not intended as endorsement.