Search for drugs:
Typing the drug name to query
TEMSIROLIMUS INJECTION
DIR Classification
Classification:Ambiguous
Severity Score:1.0
Description in Drug Labeling: View Full Labeling: SPL in DailyMed | PDF
- CLINICAL PHARMACOLOGY
- Pharmacodynamics
- Effects on Electrocardiogram: There were no clinically relevant QT changes observed at the recommended dose for temsirolimus injection. In a randomized, single-blinded, crossover study, 58 healthy subjects received temsirolimus injection 25 mg, placebo, and a single oral dose of moxifloxacin 400 mg. A supratherapeutic temsirolimus injection dose was not studied in this randomized QT trial. The largest difference between the upper bound 2-sided 90% CI for the mean difference between temsirolimus injection and placebo-corrected QT interval was less than 10 ms. In a different trial in 69 patients with a hematologic malignancy, temsirolimus injection doses up to 175 mg were studied. No patient with a normal QTcF at baseline had an increase in QTcF >60 ms. Additionally, there were no patients with a QTcF interval greater than 500 ms.
Postmarketing Surveillance
Contingency Table:
Current Drug
Other Drugs
QT Prolongation
0
24092
Other ADRs
8479
38373108
Odds Ratio = 0.0
Drug Property Information
ATC Code(s):
- L01EG01 - temsirolimus injection
- L01EG0 -
- L01EG -
- L01E -
- L01 - ANTINEOPLASTIC AGENTS
- L - ANTINEOPLASTIC AND IMMUNOMODULATING AGENTS
Active Ingredient:TEMSIROLIMUS
Active Ingredient UNII:624KN6GM2T
Drugbank ID:DB06287
PubChem Compound:23724530
CTD ID: C401859
PharmGKB:PA164746890
CAS Number:162635-04-3
Dosage Form(s):kit
Route(s) Of Administrator:intravenous
Daily Dose:
Chemical Structure: 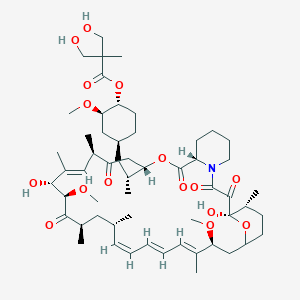

SMILE Code:
OCC(C)(CO)C(=O)O[C@@H]1CC[C@@H](C[C@@H](C)[C@]2([H])CC(=O)[C@H](C)\C=C(C)\[C@@H](O)[C@@H](OC)C(=O)[C@H](C)C[C@H](C)\C=C\C=C\C=C(C)\[C@@H](OC)C[C@]3([H])CC[C@@H](C)[C@@](O)(O3)C(=O)C(=O)N3CCCC[C@H]3C(=O)O2)C[C@H]1OC
OCC(C)(CO)C(=O)O[C@@H]1CC[C@@H](C[C@@H](C)[C@]2([H])CC(=O)[C@H](C)\C=C(C)\[C@@H](O)[C@@H](OC)C(=O)[C@H](C)C[C@H](C)\C=C\C=C\C=C(C)\[C@@H](OC)C[C@]3([H])CC[C@@H](C)[C@@](O)(O3)C(=O)C(=O)N3CCCC[C@H]3C(=O)O2)C[C@H]1OC
Reference
N/A
Disclaimer:
The content of this database of QT prolongation is intended for educational and scientific research purposes only. It is not intended as a substitute for professional medical advice, diagnosis or treatment.
Any mention of commercial products is for clarification and not intended as endorsement.