Search for drugs:
Typing the drug name to query
MEFLOQUINE HYDROCHLORIDE
DIR Classification
Classification:Most-DIQT concern
Severity Score:4.0
Description in Drug Labeling: View Full Labeling: SPL in DailyMed | PDF
- WARNINGS
- QTc Interval Prolongation and Drug Interactions
- Halofantrine should not be administered with mefloquine or within 15 weeks of the last dose of mefloquine due to the risk of a potentially fatal prolongation of the QTc interval (see CLINICAL PHARMACOLOGY, PHARMACOKINETICS, ELIMINATION).
- Ketoconazole should not be administered with mefloquine or within 15 weeks of the last dose of mefloquine due to the risk of a potentially fatal prolongation of the QTc interval. Ketoconazole increases plasma concentrations and elimination half-life of mefloquine following coadministration (see CLINICAL PHARMACOLOGY, PHARMACOKINETICS, ELIMINATION and PRECAUTIONS, DRUG INTERACTIONS).
- Concomitant administration of mefloquine and quinine or quinidine may produce electrocardiographic abnormalities.
- PRECAUTIONS
- Cardiac Effects
- Parenteral studies in animals show that mefloquine, a myocardial depressant, possesses 20% of the anti-fibrillatory action of quinidine and produces 50% of the increase in the PR interval reported with quinine. The effect of mefloquine on the compromised cardiovascular system has not been evaluated. However, transitory and clinically silent ECG alterations have been reported during the use of mefloquine; alterations included sinus bradycardia, sinus arrhythmia, first degree AV-block, prolongation of the QTc interval and abnormal T waves (see also cardiovascular effects under PRECAUTIONS, DRUG INTERACTIONS and ADVERSE REACTIONS). The benefits of mefloquine therapy should be weighed against the possibility of adverse effects in patients with cardiac disease.
- [Drug Interactions]
- Halofantrine
- Halofantrine should not be administered with mefloquine or within 15 weeks of the last dose of mefloquine due to the risk of a potentially fatal prolongation of the QTc interval (see WARNINGS).
- Other Antimalarial Drugs
- Concomitant administration of mefloquine and other related antimalarial compounds (e.g., quinine, quinidine and chloroquine) may produce electrocardiographic abnormalities and increase the risk of convulsions (see WARNINGS). If these drugs are to be used in the initial treatment of severe malaria, mefloquine administration should be delayed at least 12 hours after the last dose. Clinically significant QTc prolongation has not been found with mefloquine alone.
- Ketoconazole (Potent Inhibitor of CYP3A4)
- Coadministration of a single 500 mg oral dose of mefloquine with 400 mg of ketoconazole once daily for 10 days in 8 healthy volunteers resulted in an increase in the mean Cmax and AUC of mefloquine by 64% and 79%, respectively, and an increase in the mean elimination half-life of mefloquine from 322 hours to 448 hours. Ketoconazole should not be administered with mefloquine or within 15 weeks of the last dose of mefloquine due to the risk of a potentially fatal prolongation of the QTc interval (see WARNINGS).
- Other Drugs that Prolong the QTc Interval
- Coadministration of other drugs known to alter cardiac conduction (e.g., anti-arrhythmic or beta-adrenergic blocking agents, calcium channel blockers, antihistamines or H1-blocking agents, tricyclic antidepressants and phenothiazines) might also contribute to a prolongation of the QTc interval. There are no data that conclusively establish whether the concomitant administration of mefloquine and the above listed agents has an effect on cardiac function.
- MEDICATION GUIDE
- Mefloquine can cause serious side effects, including:
- [Heart Problems]
- Do not take halofantrine (used to treat malaria) or ketoconazole (used for fungal infections) with
- mefloquine or within 15 weeks of your last dose of mefloquine. You may get serious heart
- problems (problems with the electrical system of your heart called QT prolongation) that can lead
- to death. Do not take quinine (Qualaquin) or quinidine (used to treat malaria or irregular heart
- beat) with mefloquine. You may get serious heart problems.
- Mefloquine can cause serious side effects,including:
- [Heart problems]
- Do not take halofantrine (used to treat malaria) or ketoconazole (used for fungal infections) with mefloquine or within 15 weeks of your last dose of mefloquine. You may get serious heart problems that can lead to death. Do not take quinine (Qualaquin) or quinidine (used to treat malaria or irregular heart beat) with mefloquine. You may get serious heart problems. Mefloquine may cause serious problems with the electrical system of your heart, called QT prolongation.
Postmarketing Surveillance
Contingency Table:
Current Drug
Other Drugs
QT Prolongation
7
24085
Other ADRs
7387
38374200
Odds Ratio = 1.51
Drug Property Information
ATC Code(s):
- P01BC02 - mefloquine hydrochloride
- P01BC0 -
- P01BC - Methanolquinolines
- P01B - ANTIMALARIALS
- P01 - ANTIPROTOZOALS
- P - "ANTIPARASITIC PRODUCTS, INSECTICIDES AND REPELLENTS"
Active Ingredient:MEFLOQUINE HYDROCHLORIDE
Active Ingredient UNII:5Y9L3636O3
Drugbank ID:DB00358
PubChem Compound:4046
CTD ID:D015767
PharmGKB:PA450348
CAS Number:53230-10-7
Dosage Form(s):tablet
Route(s) Of Administrator:oral
Daily Dose:
- 1000.0 mg/day P01BC02
Chemical Structure: 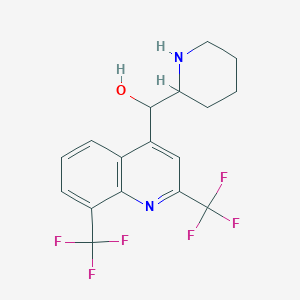

SMILE Code:
OC(C1CCCCN1)C1=CC(=NC2=C1C=CC=C2C(F)(F)F)C(F)(F)F
OC(C1CCCCN1)C1=CC(=NC2=C1C=CC=C2C(F)(F)F)C(F)(F)F
Reference
N/A
Disclaimer:
The content of this database of QT prolongation is intended for educational and scientific research purposes only. It is not intended as a substitute for professional medical advice, diagnosis or treatment.
Any mention of commercial products is for clarification and not intended as endorsement.