Search for drugs:
Typing the drug name to query
NICARDIPINE HYDROCHLORIDE
DIR Classification
Classification:Ambiguous
Severity Score:1.0
Description in Drug Labeling: View Full Labeling: SPL in DailyMed | PDF
- CLINICAL PHARMACOLOGY
- Pharmacodynamics
- Electrophysiologic Effects
- In general, no detrimental effects on the cardiac conduction system have been seen with Cardene I.V. During acute electrophysiologic studies, it increased heart rate and prolonged the corrected QT interval to a minor degree. It did not affect sinus node recovery or SA conduction times. The PA, AH, and HV intervals* or the functional and effective refractory periods of the atrium were not prolonged. The relative and effective refractory periods of the His-Purkinje system were slightly shortened.
Postmarketing Surveillance
Contingency Table:
Current Drug
Other Drugs
QT Prolongation
2
24090
Other ADRs
2995
38378592
Odds Ratio = 1.064
Drug Property Information
ATC Code(s):
- C08CA04 - nicardipine hydrochloride
- C08CA0 -
- C08CA - Dihydropyridine derivatives
- C08C - SELECTIVE CALCIUM CHANNEL BLOCKERS WITH MAINLY VASCULAR EFFECTS
- C08 - CALCIUM CHANNEL BLOCKERS
- C - CARDIOVASCULAR SYSTEM
Active Ingredient:NICARDIPINE HYDROCHLORIDE
Active Ingredient UNII:K5BC5011K3
Drugbank ID:DB00622
PubChem Compound:4474
CTD ID:D009529
PharmGKB:PA450620
CAS Number:55985-32-5
Dosage Form(s):injection, solution
Route(s) Of Administrator:intravenous
Daily Dose:
- 90.0 mg/day C08CA04
Chemical Structure: 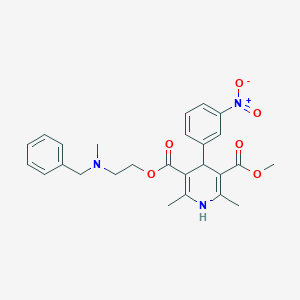

SMILE Code:
COC(=O)C1=C(C)NC(C)=C(C1C1=CC(=CC=C1)[N+]([O-])=O)C(=O)OCCN(C)CC1=CC=CC=C1
COC(=O)C1=C(C)NC(C)=C(C1C1=CC(=CC=C1)[N+]([O-])=O)C(=O)OCCN(C)CC1=CC=CC=C1
Reference
N/A
Disclaimer:
The content of this database of QT prolongation is intended for educational and scientific research purposes only. It is not intended as a substitute for professional medical advice, diagnosis or treatment.
Any mention of commercial products is for clarification and not intended as endorsement.