Search for drugs:
Typing the drug name to query
ROMIDEPSIN
DIR Classification
Classification:Ambiguous
Severity Score:1.0
Description in Drug Labeling: View Full Labeling: SPL in DailyMed | PDF
- WARNINGS AND PRECAUTIONS
- Electrocardiographic Changes
- Several treatment-emergent morphological changes in ECGs (including T-wave and ST-segment changes) have been reported in clinical studies. The clinical significance of these changes is unknown [see Adverse Reactions (6.1)].
- In patients with congenital long QT syndrome, patients with a history of significant cardiovascular disease, and patients taking anti-arrhythmic medicines or medicinal products that lead to significant QT prolongation, consider cardiovascular monitoring of ECGs at baseline and periodically during treatment.
- Confirm that potassium and magnesium levels are within normal range before administration of Romidepsin Injection [see Adverse Reactions (6.1)].
- ADVERSE REACTIONS
- Clinical Trials Experience
- Discontinuations
- Discontinuation due to an adverse event occurred in 21% of patients in Study 1 and 11% in Study 2. Discontinuations occurring in at least 2% of patients in either study included infection, fatigue, dyspnea, QT prolongation, and hypomagnesemia.
- CLINICAL PHARMACOLOGY
- Pharmacodynamics
- Cardiac Electrophysiology
- At doses of 14 mg/m2 as a 4-hour intravenous infusion and at doses of 8 (0.57 times the recommended dose), 10 (0.71 times the recommended dose) or 12 (0.86 times the recommended dose) mg/m2 as a 1-hour infusion, no large changes in the mean QTc interval (>20 milliseconds) from baseline based on Fridericia correction method were detected. Small increase in mean QT interval (< 10 milliseconds) and mean QT interval increase between 10 to 20 milliseconds cannot be excluded.
- Romidepsin was associated with a delayed concentration-dependent increase in heart rate in patients with advanced cancer with a maximum mean increase in heart rate of 20 beats per minute occurring at the 6-hour time point after start of romidepsin infusion for patients receiving 14 mg/m2 as a 4-hour infusion.
Postmarketing Surveillance
Contingency Table:
Current Drug
Other Drugs
QT Prolongation
21
24071
Other ADRs
2537
38379050
Odds Ratio = 13.198
Drug Property Information
ATC Code(s):
- L01XH02 - romidepsin
- L01XH -
- L01X - OTHER ANTINEOPLASTIC AGENTS
- L01 - ANTINEOPLASTIC AGENTS
- L - ANTINEOPLASTIC AND IMMUNOMODULATING AGENTS
Active Ingredient:ROMIDEPSIN
Active Ingredient UNII:CX3T89XQBK
Drugbank ID:DB06176
PubChem Compound:57515973
CTD ID: C087123
PharmGKB:PA166161306
CAS Number:128517-07-7
Dosage Form(s):injection, solution, concentrate
Route(s) Of Administrator:intravenous
Daily Dose:
Chemical Structure: 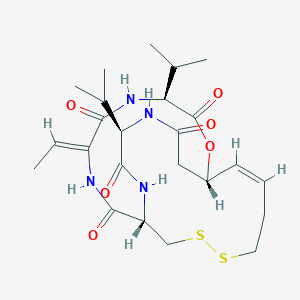

SMILE Code:
C\C=C1/NC(=O)[C@H]2CSSCC\C=C\[C@H](CC(=O)N[C@H](C(C)C)C(=O)N2)OC(=O)[C@@H](NC1=O)C(C)C
C\C=C1/NC(=O)[C@H]2CSSCC\C=C\[C@H](CC(=O)N[C@H](C(C)C)C(=O)N2)OC(=O)[C@@H](NC1=O)C(C)C
Reference
1: Combined effect of Vacc-4x, recombinant human granulocyte macrophage colony-stimulating factor vaccination, and romidepsin on the HIV-1 reservoir (REDUC): a single-arm, phase 1B/2A trial.
[Leth Steffen,Schleimann Mariane H,Nissen Sara K,Højen Jesper F,Olesen Rikke,Graversen Mette E,Jørgensen Sofie,Kjær Anne Sofie,Denton Paul W,Mørk Alejandra,Sommerfelt Maja A,Krogsgaard Kim,Østergaard Lars,Rasmussen Thomas A,Tolstrup Martin,Søgaard Ole Schmeltz]Lancet HIV,2016 Oct;3(10):e463-72. PMID: 27658863
2: Romidepsin for the Treatment of Peripheral T-Cell Lymphoma.
[Iyer Swaminathan P,Foss Francine F]Oncologist,2015 Sep;20(9):1084-91. PMID: 26099743
3: Impact of ABCB1 allelic variants on QTc interval prolongation.
[Sissung Tristan M,Gardner Erin R,Piekarz Richard L,Howden Reuben,Chen Xiaohong,Woo Sukyung,Franke Ryan,Clark James A,Miller-DeGraff Laura,Steinberg Seth M,Venzon David,Liewehr David,Kleeberger Steven R,Bates Susan E,Price Douglas K,Rosing Douglas R,Cabell Christopher,Sparreboom Alex,Figg William D]Clin Cancer Res,2011 Feb 15;17(4):937-46. PMID: 21106724
Disclaimer:
The content of this database of QT prolongation is intended for educational and scientific research purposes only. It is not intended as a substitute for professional medical advice, diagnosis or treatment.
Any mention of commercial products is for clarification and not intended as endorsement.