Search for drugs:
Typing the drug name to query
LEXISCAN(R) (REGADENOSON)
DIR Classification
Classification:Ambiguous
Severity Score:1.0
Description in Drug Labeling: View Full Labeling: SPL in DailyMed | PDF
- ADVERSE REACTIONS
- Post-Marketing Experience
- Cardiovascular
- Myocardial infarction, cardiac arrest, ventricular arrhythmias, supraventricular tachyarrhythmias including atrial fibrillation with rapid ventricular response (new-onset or recurrent), atrial flutter, heart block (including third-degree block), asystole, marked hypertension, symptomatic hypotension in association with transient ischemic attack, acute coronary syndrome (ACS), seizures and syncope [see Warnings and Precautions ( 5-5.1), ( 5-5.2), ( 5-5.3), ( 5-5.5), ( 5-5.6) and ( 5-5.8)] have been reported. Some events required intervention with fluids and/or aminophylline [see Overdosage ( 10)]. QTc prolongation shortly after LEXISCAN administration has been reported.
Postmarketing Surveillance
Contingency Table:
Current Drug
Other Drugs
QT Prolongation
0
24092
Other ADRs
0
38381587
Odds Ratio = N/A
Drug Property Information
ATC Code(s):
- C01EB21 - lexiscan(r) (regadenoson)
- C01EB - Other cardiac preparations
- C01E - OTHER CARDIAC PREPARATIONS
- C01 - CARDIAC THERAPY
- C - CARDIOVASCULAR SYSTEM
Active Ingredient:REGADENOSON
Active Ingredient UNII:2XLN4Y044H
Drugbank ID:DB06213
PubChem Compound:219024
CTD ID:C430916
PharmGKB:PA166129536
CAS Number:313348-27-5
Dosage Form(s):injection, solution
Route(s) Of Administrator:intravenous
Daily Dose:
- 0.4 mg/day C01EB21
Chemical Structure: 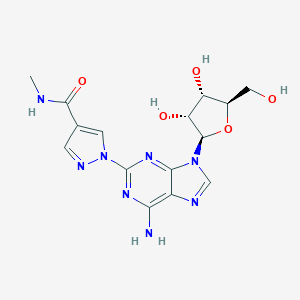

SMILE Code:
CNC(=O)C1=CN(N=C1)C1=NC2=C(N=CN2[C@@H]2O[C@H](CO)[C@@H](O)[C@H]2O)C(N)=N1
CNC(=O)C1=CN(N=C1)C1=NC2=C(N=CN2[C@@H]2O[C@H](CO)[C@@H](O)[C@H]2O)C(N)=N1
Reference
N/A
Disclaimer:
The content of this database of QT prolongation is intended for educational and scientific research purposes only. It is not intended as a substitute for professional medical advice, diagnosis or treatment.
Any mention of commercial products is for clarification and not intended as endorsement.