Search for drugs:
Typing the drug name to query
POSACONAZOLE
DIR Classification
Classification:Most-DIQT concern
Severity Score:4.0
Description in Drug Labeling: View Full Labeling: SPL in DailyMed | PDF
- WARNINGS AND PRECAUTIONS
- Arrhythmias and QT Prolongation
- Some azoles, including posaconazole, have been associated with prolongation of the QT interval on the electrocardiogram. In addition, cases of torsades de pointes have been reported in patients taking posaconazole.
- Results from a multiple time-matched ECG analysis in healthy volunteers did not show any increase in the mean of the QTc interval. Multiple, time-matched ECGs collected over a 12-hour period were recorded at baseline and steady-state from 173 healthy male and female volunteers (18 to 85 years of age) administered posaconazole oral suspension 400 mg BID with a high-fat meal. In this pooled analysis, the mean QTc (Fridericia) interval change from baseline was –5 msec following administration of the recommended clinical dose. A decrease in the QTc(F) interval (–3 msec) was also observed in a small number of subjects (n=16) administered placebo. The placebo-adjusted mean maximum QTc(F) interval change from baseline was <0 msec (–8 msec). No healthy subject administered posaconazole had a QTc(F) interval ≥500 msec or an increase ≥60 msec in their QTc(F) interval from baseline.
- Posaconazole should be administered with caution to patients with potentially proarrhythmic conditions. Do not administer with drugs that are known to prolong the QTc interval and are metabolized through CYP3A4 [see Contraindications (4.3) and Drug Interactions (7.2)].
- DRUG INTERACTIONS
- CYP3A4 Substrates
- Concomitant administration of posaconazole with CYP3A4 substrates such as pimozide and quinidine may result in increased plasma concentrations of these drugs, leading to QTc prolongation and cases of torsades de pointes. Therefore, posaconazole is contraindicated with these drugs [see Contraindications (4.3) and Warnings and Precautions (5.2)].
- CONTRAINDICATIONS
- QT Prolongation with Concomitant Use with CYP3A4 Substrates
- Posaconazole is contraindicated with CYP3A4 substrates that prolong the QT interval. Concomitant administration of posaconazole with the CYP3A4 substrates, pimozide and quinidine may result in increased plasma concentrations of these drugs, leading to QTc prolongation and cases of torsades de pointes [see Warnings and Precautions (5.2) and Drug Interactions (7.2)].
- ADVERSE REACTIONS
- The following serious and otherwise important adverse reactions are discussed in detail in another section of the labeling:
- Arrhythmias and QT Prolongation [see Warnings and Precautions (5.2)]
- [CYP3A4 Substrates]
- Concomitant administration of posaconazole with CYP3A4 substrates such as pimozide and quinidine may result in increased plasma concentrations of these drugs, leading to QTc prolongation and cases of torsades de pointes. Therefore, posaconazole is contraindicated with these drugs [see Contraindications (4.3) and Warnings and Precautions (5.2)].
- PATIENT COUNSELING INFORMATION
- Drug Interactions
- Advise patients to inform their physician immediately if they:
- develop severe diarrhea or vomiting.
- are currently taking drugs that are known to prolong the QTc interval and are metabolized through CYP3A4.
- Posaconazole delayed-release tablets may cause serious side effects, including:
- problems with the electrical system of your heart (arrhythmias and QTc prolongation). Certain medicines used to treat fungus called azoles, including posaconazole, the active ingredient in posaconazole delayed-release tablets, may cause heart rhythm problems. People who have certain heart problems or who take certain medicines have a higher chance for this problem. Tell your healthcare provider right away if your heartbeat becomes fast or irregular.
Postmarketing Surveillance
Contingency Table:
Current Drug
Other Drugs
QT Prolongation
36
24056
Other ADRs
5894
38375693
Odds Ratio = 9.744
Drug Property Information
ATC Code(s):
- J02AC04 - posaconazole
- J02AC - Triazole derivatives
- J02A - ANTIMYCOTICS FOR SYSTEMIC USE
- J02 - ANTIMYCOTICS FOR SYSTEMIC USE
- J - ANTIINFECTIVES FOR SYSTEMIC USE
Active Ingredient:POSACONAZOLE
Active Ingredient UNII:6TK1G07BHZ
Drugbank ID:DB01263
PubChem Compound:468595
CTD ID:C101425
PharmGKB:PA151958574
CAS Number:171228-49-2
Dosage Form(s):tablet, delayed release
Route(s) Of Administrator:oral
Daily Dose:
- 300.0 mg/day J02AC04
Chemical Structure: 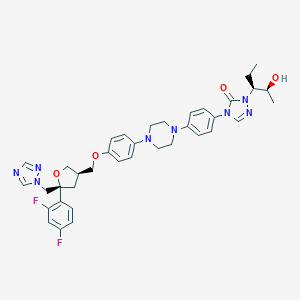

SMILE Code:
[H][C@@](C)(O)[C@]([H])(CC)N1N=CN(C1=O)C1=CC=C(C=C1)N1CCN(CC1)C1=CC=C(OC[C@]2([H])CO[C@](CN3C=NC=N3)(C2)C2=C(F)C=C(F)C=C2)C=C1
[H][C@@](C)(O)[C@]([H])(CC)N1N=CN(C1=O)C1=CC=C(C=C1)N1CCN(CC1)C1=CC=C(OC[C@]2([H])CO[C@](CN3C=NC=N3)(C2)C2=C(F)C=C(F)C=C2)C=C1
Reference
1: Characterization of Therapeutic Drug Monitoring Practices of Voriconazole and Posaconazole at a Pediatric Hospital.
[Duehlmeyer Stephanie,Klockau Christopher,Yu Diana,Rouch Jamie]J Pediatr Pharmacol Ther,2021;26(1):26-32. PMID: 33424497
2: Comparative evaluation of isavuconazonium sulfate, voriconazole, and posaconazole for the management of invasive fungal infections in an academic medical center.
[Van Matre Edward T,Evans Shelby L,Mueller Scott W,MacLaren Robert,Fish Douglas N,Kiser Tyree H]Ann Clin Microbiol Antimicrob,2019 Mar 20;18(1):13. PMID: 30894179
3: Tolerability of isavuconazole after posaconazole toxicity in leukaemia patients.
[DiPippo Adam J,Rausch Caitlin R,Kontoyiannis Dimitrios P]Mycoses,2019 Jan;62(1):81-86. PMID: 30230043
4: Treatment of Chronic Pulmonary Aspergillosis: Current Standards and Future Perspectives.
[Alastruey-Izquierdo Ana,Cadranel Jacques,Flick Holger,Godet Cendrine,Hennequin Christophe,Hoenigl Martin,Kosmidis Chris,Lange Christoph,Munteanu Oxana,Page Iain,Salzer Helmut J F,on behalf of CPAnet]Respiration,2018;96(2):159-170. PMID: 29982245
5: Persistence of a Posaconazole-Mediated Drug-Drug Interaction With Ranolazine After Cessation of Posaconazole Administration: Impact of Obesity and Implications for Patient Safety.
[Chow Christina R,Harmatz Jerold S,Ryan Michael J,Greenblatt David J]J Clin Pharmacol,2018 Nov;58(11):1436-1442. PMID: 29749631
6: Torsade de pointes and systemic azole antifungal agents: Analysis of global spontaneous safety reports.
[Salem M,Reichlin T,Fasel D,Leuppi-Taegtmeyer A]Glob Cardiol Sci Pract,2017 Jun 30;2017(2):11. PMID: 29644223
7: Posaconazole liquid vs tablet formulation in lung transplant recipients.
[Stelzer D,Weber A,Ihle F,Matthes S,Ceelen F,Zimmermann G,Kneidinger N,Schramm R,Winter H,Zoller M,Vogeser M,Behr J,Neurohr C]Mycoses,2018 Mar;61(3):186-194. PMID: 29110351
8: Evaluation of Serum Posaconazole Concentrations in Patients with Hematological Malignancies Receiving Posaconazole Suspension Compared to the Delayed-Release Tablet Formulation.
[Belling Morgan,Kanate Abraham S,Shillingburg Alexandra,Lu Xiaoxiao,Wen Sijin,Shah Nilay,Craig Michael,Cumpston Aaron]Leuk Res Treatment,2017;2017:3460892. PMID: 28695013
9: Multicentre study of posaconazole delayed-release tablet serum level and association with hepatotoxicity and QTc prolongation.
[Pettit Natasha N,Miceli Marisa H,Rivera Christina G,Narayanan Prasanna P,Perissinotti Anthony J,Hsu Meier,Delacruz Jennifer,Gedrimaite Zivile,Han Zhe,Steinbeck Jennifer,Pisano Jennifer,Seo Susan K,Paskovaty Alla]J Antimicrob Chemother,2017 Aug 1;72(8):2355-2358. PMID: 28475803
10: Role of isavuconazole in the treatment of invasive fungal infections.
[Wilson Dustin T,Dimondi V Paul,Johnson Steven W,Jones Travis M,Drew Richard H]Ther Clin Risk Manag,2016 Aug 3;12:1197-206. PMID: 27536124
Disclaimer:
The content of this database of QT prolongation is intended for educational and scientific research purposes only. It is not intended as a substitute for professional medical advice, diagnosis or treatment.
Any mention of commercial products is for clarification and not intended as endorsement.