Search for drugs:
Typing the drug name to query
OZANIMOD HYDROCHLORIDE
DIR Classification
Classification:Moderate-DIQT concern
Severity Score:3.0
Description in Drug Labeling: View Full Labeling: SPL in DailyMed | PDF
- DRUG INTERACTIONS
- Anti-Arrhythmic Drugs, QT Prolonging Drugs, Drugs That may Decrease Heart Rate
- ZEPOSIA has not been studied in patients taking QT prolonging drugs.
- Class Ia (e.g., quinidine, procainamide) and Class III (e.g., amiodarone, sotalol) anti-arrhythmic drugs have been associated with cases of Torsades de Pointes in patients with bradycardia. If treatment with ZEPOSIA is considered, advice from a cardiologist should be sought.
- Because of the potential additive effects on heart rate, treatment with ZEPOSIA should generally not be initiated in patients who are concurrently treated with QT prolonging drugs with known arrhythmogenic properties [see WARNINGS AND PRECAUTIONS (5.2)]. If treatment initiation with ZEPOSIA is considered in patients on QT prolonging drugs, advice from a cardiologist should be sought.
- CLINICAL PHARMACOLOGY
- Pharmacodynamics
- Cardiac Electrophysiology
- Following a 14-day titration regimen of once daily doses of ozanimod 0.23 mg for 4 days, 0.46 mg for 3 days, 0.92 mg for 3 days, and 1.84 mg (2 times the maximum approved recommended dose) for 4 days in healthy subjects, ZEPOSIA did not prolong the QTc interval to any clinically relevant extent [see WARNINGS AND PRECAUTIONS (5.2)].
Postmarketing Surveillance
Contingency Table:
Current Drug
Other Drugs
QT Prolongation
0
24092
Other ADRs
0
38381587
Odds Ratio = N/A
Drug Property Information
ATC Code(s):
- L04AA38 - ozanimod hydrochloride
- L04AA - Selective immunosuppressants
- L04A - IMMUNOSUPPRESSANTS
- L04 - IMMUNOSUPPRESSANTS
- L - ANTINEOPLASTIC AND IMMUNOMODULATING AGENTS
Active Ingredient:OZANIMOD HYDROCHLORIDE
Active Ingredient UNII:3UPR33JAAM
Drugbank ID:DB12612
PubChem Compound:52938427
CTD ID:C000607776
CAS Number:1306760-87-1
Dosage Form(s):capsule; kit
Route(s) Of Administrator:oral
Daily Dose:
Chemical Structure: 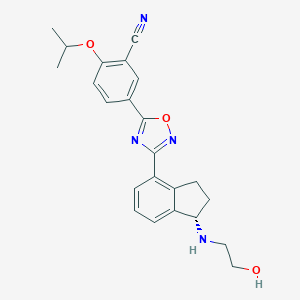

SMILE Code:
CC(C)OC1=C(C=C(C=C1)C1=NC(=NO1)C1=C2CC[C@H](NCCO)C2=CC=C1)C#N
CC(C)OC1=C(C=C(C=C1)C1=NC(=NO1)C1=C2CC[C@H](NCCO)C2=CC=C1)C#N
Reference
N/A
Disclaimer:
The content of this database of QT prolongation is intended for educational and scientific research purposes only. It is not intended as a substitute for professional medical advice, diagnosis or treatment.
Any mention of commercial products is for clarification and not intended as endorsement.