Search for drugs:
Typing the drug name to query
IVABRADINE
DIR Classification
Classification:Most-DIQT concern
Severity Score:4.0
Description in Drug Labeling: View Full Labeling: SPL in DailyMed | PDF
- WARNINGS AND PRECAUTIONS
- Bradycardia and Conduction Disturbances
- Adult Patients
- Bradycardia, sinus arrest, and heart block have occurred with Corlanor. The rate of bradycardia was 6.0% per patient-year in patients treated with Corlanor (2.7% symptomatic; 3.4% asymptomatic) and 1.3% per patient-year in patients treated with placebo. Risk factors for bradycardia include sinus node dysfunction, conduction defects (e.g., 1st or 2nd degree atrioventricular block, bundle branch block), ventricular dyssynchrony, and use of other negative chronotropes (e.g., digoxin, diltiazem, verapamil, amiodarone). Bradycardia may increase the risk of QT prolongation which may lead to severe ventricular arrhythmias, including torsade de pointes, especially in patients with risk factors such as use of QTc prolonging drugs [see Adverse Reactions (6.2)].
- CLINICAL PHARMACOLOGY
- Pharmacodynamics
- Corlanor does not have negative inotropic effects. Ivabradine increases the uncorrected QT interval with heart rate slowing but does not cause rate-corrected prolongation of QT.
Postmarketing Surveillance
Contingency Table:
Current Drug
Other Drugs
QT Prolongation
24
24068
Other ADRs
4907
38376680
Odds Ratio = 7.799
Drug Property Information
ATC Code(s):
- C01EB17 - ivabradine
- C01EB - Other cardiac preparations
- C01E - OTHER CARDIAC PREPARATIONS
- C01 - CARDIAC THERAPY
- C - CARDIOVASCULAR SYSTEM
Active Ingredient:IVABRADINE HYDROCHLORIDE
Active Ingredient UNII:TP19837BZK
Drugbank ID:DB09083
PubChem Compound:132999
CTD ID:D000077550
PharmGKB:PA166123415
CAS Number:155974-00-8
Dosage Form(s):solution; tablet, film coated
Route(s) Of Administrator:oral
Daily Dose:
- 10.0 mg/day C01EB17
Chemical Structure: 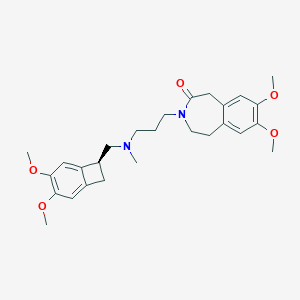

SMILE Code:
COC1=C(OC)C=C2[C@@H](CN(C)CCCN3CCC4=CC(OC)=C(OC)C=C4CC3=O)CC2=C1
COC1=C(OC)C=C2[C@@H](CN(C)CCCN3CCC4=CC(OC)=C(OC)C=C4CC3=O)CC2=C1
Reference
1: Ivabradine is as effective as metoprolol in the prevention of ventricular arrhythmias in acute non-reperfused myocardial infarction in the rat.
[Marciszek Mariusz,Paterek Aleksandra,Oknińska Marta,Mackiewicz Urszula,Mączewski Michał]Sci Rep,2020 Sep 14;10(1):15027. PMID: 32929098
2: Ivabradine-Induced Torsade de Pointes in Patients with Heart Failure Reduced Ejection Fraction.
[Jang Ji-Hun,Kwon Sung Woo,Lee Man-Jong,Ko Kyu-Yong,Park Jin-Hee,Yoon Gwang-Seok,Choi Seong-Huan,Beak Yong-Soo,Park Sang-Don,Shin Sung-Hee,Woo Seong-Ill,Kim Dae-Hyeok,Kwan Jun]Int Heart J,2020 Sep 29;61(5):1044-1048. PMID: 32921663
3: Ivabradine Aggravates the Proarrhythmic Risk in Experimental Models of Long QT Syndrome.
[Frommeyer Gerrit,Weller Jan,Ellermann Christian,Leitz Patrick,Kochhäuser Simon,Lange Philipp S,Dechering Dirk G,Eckardt Lars]Cardiovasc Toxicol,2019 Apr;19(2):129-135. PMID: 30238354
4: Human Induced Pluripotent Stem Cell-Derived Engineered Heart Tissue as a Sensitive Test System for QT Prolongation and Arrhythmic Triggers.
[Lemoine Marc D,Krause Tobias,Koivumäki Jussi T,Prondzynski Maksymilian,Schulze Mirja L,Girdauskas Evaldas,Willems Stephan,Hansen Arne,Eschenhagen Thomas,Christ Torsten]Circ Arrhythm Electrophysiol,2018 Jul;11(7):e006035. PMID: 29925535
5: hERG potassium channel inhibition by ivabradine may contribute to QT prolongation and risk of torsades de pointes.
[Hancox Jules C,Melgari Dario,Dempsey Christopher E,Brack Kieran E,Mitcheson John,Ng G André]Ther Adv Drug Saf,2015 Aug;6(4):177-9. PMID: 26301071
6: Slow junctional rhythm, QTc prolongation and transient torsades de-pointes following combined use of Ivabradine, Diltiazem and Ranolazine.
[Mittal S R]J Assoc Physicians India,2014 May;62(5):426-7. PMID: 25438492
7: Combined actions of ivabradine and ranolazine reduce ventricular rate during atrial fibrillation.
[Verrier Richard L,Silva Ana F G,Bonatti Rodolfo,Batatinha Julio A P,Nearing Bruce D,Liu Gongxin,Rajamani Sridharan,Zeng Dewan,Belardinelli Luiz]J Cardiovasc Electrophysiol,2015 Mar;26(3):329-35. PMID: 25346368
8: [Pharmacotherapy of cardiac arrhythmias in women--what do we know, do we have a choice?].
[Klocek Marek,Skrzek Agnieszka,Czarnecka Danuta]Przegl Lek,2014;71(3):155-9. PMID: 25154213
9: Novel therapeutic approaches to treating chronic angina in the setting of chronic ischemic heart disease.
[Chaitman Bernard R,Sano Junko]Clin Cardiol,2007 Feb;30(2 Suppl 1):I25-30. PMID: 18373327
Disclaimer:
The content of this database of QT prolongation is intended for educational and scientific research purposes only. It is not intended as a substitute for professional medical advice, diagnosis or treatment.
Any mention of commercial products is for clarification and not intended as endorsement.