Search for drugs:
Typing the drug name to query
CLARITHROMYCIN
DIR Classification
Classification:Most-DIQT concern
Severity Score:4.0
Description in Drug Labeling: View Full Labeling: SPL in DailyMed | PDF
- WARNINGS AND PRECAUTIONS
- QT Prolongation
- Clarithromycin has been associated with prolongation of the QT interval and infrequent cases of arrhythmia. Cases of torsades de pointes have been spontaneously reported during postmarketing surveillance in patients receiving clarithromycin. Fatalities have been reported.
- Avoid clarithromycin in the following patients:
- patients with known prolongation of the QT interval, ventricular cardiac arrhythmia, including torsades de pointes
- patients receiving drugs known to prolong the QT interval [see also Contraindications (4.2)]
- patients with ongoing proarrhythmic conditions such as uncorrected hypokalemia or hypomagnesemia, clinically significant bradycardia and in patients receiving Class IA (quinidine, procainamide) or Class III (dofetilide, amiodarone, sotalol) antiarrhythmic agents.
- Elderly patients may be more susceptible to drug-associated effects on the QT interval [see Use in Specific Populations (8.5)] .
- [Serious Adverse Reactions Due to Concomitant Use with Other Drugs]
- Quetiapine: Use quetiapine and clarithromycin concomitantly with caution. Co-administration could result in increased quetiapine exposure and quetiapine related toxicities such as somnolence, orthostatic hypotension, altered state of consciousness, neuroleptic malignant syndrome, and QT prolongation. Refer to quetiapine prescribing information for recommendations on dose reduction if co-administered with CYP3A4 inhibitors such as clarithromycin [see Drug Interactions (7)] .
- DRUG INTERACTIONS
- Procainamide Not Recommended Disopyramide, Quinidine: There have been postmarketing reports of torsades de pointes occurring with concurrent use of clarithromycin and quinidine or disopyramide. Electrocardiograms should be monitored for QTc prolongation during coadministration of clarithromycin with these drugs [see Warnings and Precautions (5.3)] .
- Serum concentrations of these medications should also be monitored. There have been spontaneous or published reports of CYP3A based interactions of clarithromycin with disopyramide and quinidine.
- Quetiapine Quetiapine: Quetiapine is a substrate for CYP3A4, which is inhibited by clarithromycin. Co-administration with clarithromycin could result in increased quetiapine exposure and possible quetiapine related toxicities. There have been postmarketing reports of somnolence, orthostatic hypotension, altered state of consciousness, neuroleptic malignant syndrome, and QT prolongation during concomitant administration. Refer to quetiapine prescribing information for recommendations on dose reduction if co-administered with CYP3A4 inhibitors such as clarithromycin.
- CONTRAINDICATIONS
- Cardiac Arrhythmias
- Concomitant administration of clarithromycin with cisapride and pimozide is contraindicated [see Drug Interactions (7)] .
- There have been postmarketing reports of drug interactions when clarithromycin is co-administered with cisapride or pimozide, resulting in cardiac arrhythmias (QT prolongation, ventricular tachycardia, ventricular fibrillation, and torsades de pointes) most likely due to inhibition of metabolism of these drugs by clarithromycin. Fatalities have been reported.
- ADVERSE REACTIONS
- QT Prolongation [see Warnings and Precautions (5.2)]
- Cardiac Disorders: Electrocardiogram QT prolonged, cardiac arrest, atrial fibrillation, extrasystoles, palpitations
Postmarketing Surveillance
Contingency Table:
Current Drug
Other Drugs
QT Prolongation
210
23882
Other ADRs
44515
38337072
Odds Ratio = 7.573
Drug Property Information
ATC Code(s):
- J01FA09 - clarithromycin
- J01FA - Macrolides
- J01F - "MACROLIDES, LINCOSAMIDES AND STREPTOGRAMINS"
- J01 - ANTIBACTERIALS FOR SYSTEMIC USE
- J - ANTIINFECTIVES FOR SYSTEMIC USE
Active Ingredient:CLARITHROMYCIN
Active Ingredient UNII:H1250JIK0A
Drugbank ID:DB01211
PubChem Compound:84029
CTD ID:D017291
PharmGKB:PA449028
CAS Number:81103-11-9
Dosage Form(s):tablet, film coated
Route(s) Of Administrator:oral
Daily Dose:
- 500.0 mg/day J01FA09
Chemical Structure: 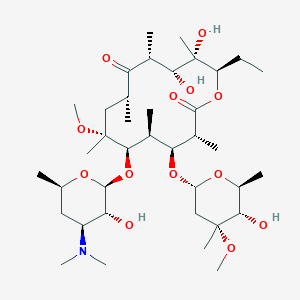

SMILE Code:
[H][C@@]1(C[C@@](C)(OC)[C@@H](O)[C@H](C)O1)O[C@H]1[C@H](C)[C@@H](O[C@]2([H])O[C@H](C)C[C@@H]([C@H]2O)N(C)C)[C@@](C)(C[C@@H](C)C(=O)[C@H](C)[C@@H](O)[C@](C)(O)[C@@H](CC)OC(=O)[C@@H]1C)OC
[H][C@@]1(C[C@@](C)(OC)[C@@H](O)[C@H](C)O1)O[C@H]1[C@H](C)[C@@H](O[C@]2([H])O[C@H](C)C[C@@H]([C@H]2O)N(C)C)[C@@](C)(C[C@@H](C)C(=O)[C@H](C)[C@@H](O)[C@](C)(O)[C@@H](CC)OC(=O)[C@@H]1C)OC
Reference
1: Risk of QT prolongation through drug interactions between hydroxychloroquine and concomitant drugs prescribed in real world practice.
[Choi Byung Jin,Koo Yeryung,Kim Tae Young,Chung Wou Young,Jung Yun Jung,Park Ji Eun,Lim Hong-Seok,Park Bumhee,Yoon Dukyong]Sci Rep,2021 Mar 25;11(1):6918. PMID: 33767276
2: {'sup': '1', '#text': 'A Comparative Study of Rat Urine H-NMR Metabolome Changes Presumably Arising from Isoproterenol-Induced Heart Necrosis Versus Clarithromycin-Induced QT Interval Prolongation.'}
[Dallons Matthieu,Delcourt Manon,Schepkens Corentin,Podrecca Manuel,Colet Jean-Marie]Biology (Basel),2020 May 13;9(5):98. PMID: 32414184
3: Treatment of Helicobacter pylori in Special Patient Populations.
[Nguyen Cynthia T,Davis Kyle A,Nisly Sarah A,Li Julius]Pharmacotherapy,2019 Oct;39(10):1012-1022. PMID: 31400244
4: Simplifying QT prolongation for busy clinicians.
[Grindrod Kelly A,Nagge Jeff]Can Fam Physician,2019 Apr;65(4):268-270. PMID: 30979760
5: Progress in the Consideration of Possible Sex Differences in Drug Interaction Studies.
[Naidoo Panjasaram,Chetty Manoranjenni]Curr Drug Metab,2019;20(2):114-123. PMID: 30488793
6: Appraisal of the cardiovascular risks of azithromycin: an observational analysis.
[Sutton S Scott,Hyche Stephanie,Magagnoli Joseph,Hardin James W]J Comp Eff Res,2017 Sep;6(6):509-517. PMID: 28960092
7: Clinical significance of QT-prolonging drug use in patients with MDR-TB or NTM disease.
[Yoon H-Y,Jo K-W,Nam G B,Shim T S]Int J Tuberc Lung Dis,2017 Sep 1;21(9):996-1001. PMID: 28826448
8: Time-to-Onset Analysis of Drug-Induced Long QT Syndrome Based on a Spontaneous Reporting System for Adverse Drug Events.
[Sasaoka Sayaka,Matsui Toshinobu,Hane Yuuki,Abe Junko,Ueda Natsumi,Motooka Yumi,Hatahira Haruna,Fukuda Akiho,Naganuma Misa,Hasegawa Shiori,Kinosada Yasutomi,Nakamura Mitsuhiro]PLoS One,2016 Oct 10;11(10):e0164309. PMID: 27723808
9: Drug safety of macrolide and quinolone antibiotics in a tertiary care hospital: administration of interacting co-medication and QT prolongation.
[Niedrig David,Maechler Sarah,Hoppe Liesa,Corti Natascia,Kovari Helen,Russmann Stefan]Eur J Clin Pharmacol,2016 Jul;72(7):859-67. PMID: 27023463
10: Drug-Induced QTc Interval Prolongation: A Multicenter Study to Detect Drugs and Clinical Factors Involved in Every Day Practice.
[Keller Guillermo A,Alvarez Paulino A,Ponte Marcelo L,Belloso Waldo H,Bagnes Claudia,Sparanochia Cecilia,Gonzalez Claudio D,Villa Etchegoyen M Cecilia,Diez Roberto A,Di Girolamo Guillermo]Curr Drug Saf,2016;11(1):86-98. PMID: 26537523
11: [Combination therapy with fluconazole and other QTc-prolonging drugs increase the QTc interval].
[Buch Tina,Andersen Stig Ejdrup]Ugeskr Laeger,2015 Oct 5;177(41):V04150371. PMID: 26471025
12: Toxicity of macrolide antibiotics on isolated heart mitochondria: a justification for their cardiotoxic adverse effect.
[Salimi Ahmad,Eybagi Sadaf,Seydi Enayatollah,Naserzadeh Parvaneh,Kazerouni Negar Panahi,Pourahmad Jalal]Xenobiotica,2016;46(1):82-93. PMID: 26068526
13: Multiple logistic regression analysis of risk factors in elderly pneumonia patients: QTc interval prolongation as a prognostic factor.
[Taooka Yasuyuki,Takezawa Gen,Ohe Miki,Sutani Akihisa,Isobe Takeshi]Multidiscip Respir Med,2014 Nov 22;9(1):59. PMID: 25705382
14: Erythromycin, QTc interval prolongation, and torsade de pointes: Case reports, major risk factors and illness severity.
[Hancox Jules C,Hasnain Mehrul,Vieweg W Victor R,Gysel Michael,Methot Michelle,Baranchuk Adrian]Ther Adv Infect Dis,2014 Apr;2(2):47-59. PMID: 25165555
15: Clarithromycin-Induced Torsades de Pointes.
[Chang Nai-Lun,Shah Priyank,Bikkina Mahesh,Shamoon Fayez]Am J Ther,May-Jun 2016;23(3):e955-6. PMID: 25057773
16: Hypokalemia: a potent risk for QTc prolongation in clarithromycin treated rats.
[Karmakar Sanmoy,Padman Aswathi,Swamy Mane Naga,Sen Tuhinadri]Eur J Pharmacol,2013 Jun 5;709(1-3):80-4. PMID: 23567068
17: Clarithromycin-Induced Long QT Syndrome: A Case Report.
[Cetin Mecnun,Yıldırımer Munevver,Ozen Serkan,Tanrıverdi Sema,Coskun Senol]Case Rep Med,2012;2012:634652. PMID: 22489247
18: The cardiotoxicity of macrolides: a systematic review.
[Guo Daihong,Cai Yun,Chai Dong,Liang Beibei,Bai Nan,Wang Rui]Pharmazie,2010 Sep;65(9):631-40. PMID: 21038838
19: Clarithromycin induced torsade de pointes.
[Hensey C,Keane D]Ir J Med Sci,2008 Mar;177(1):67-8. PMID: 17618400
20: Clarithromycin treatment and QT prolongation in childhood.
[Germanakis Ioannis,Galanakis Emmanouil,Parthenakis Fragiskos,Vardas Panos E,Kalmanti Maria]Acta Paediatr,2006 Dec;95(12):1694-6. PMID: 17129988
21: Mechanism-based inhibition of cytochrome P450 3A4 by therapeutic drugs.
[Zhou Shufeng,Yung Chan Sui,Cher Goh Boon,Chan Eli,Duan Wei,Huang Min,McLeod Howard L]Clin Pharmacokinet,2005;44(3):279-304. PMID: 15762770
22: Pharmacokinetic factors in the adverse cardiovascular effects of antipsychotic drugs.
[Brown Candace S,Farmer Richard G,Soberman Judith E,Eichner Samantha F]Clin Pharmacokinet,2004;43(1):33-56. PMID: 14715050
23: Characterization of the inhibitory effects of erythromycin and clarithromycin on the HERG potassium channel.
[Stanat Scott J C,Carlton Carol G,Crumb William J,Agrawal Krishna C,Clarkson Craig W]Mol Cell Biochem,2003 Dec;254(1-2):1-7. PMID: 14674677
24: Inhibition of cytochrome P450 3A: relevant drug interactions in gastroenterology.
[Sagir A,Schmitt M,Dilger K,Häussinger D]Digestion,2003;68(1):41-8. PMID: 12949438
25: Comparative effects of clarithromycin on action potential and ionic currents from rabbit isolated atrial and ventricular myocytes.
[Gluais Pascale,Bastide Michìle,Caron Jacques,Adamantidis Monique]J Cardiovasc Pharmacol,2003 Apr;41(4):506-17. PMID: 12658051
26: Prescription of QT-prolonging drugs in a cohort of about 5 million outpatients.
[Curtis Lesley H,Østbye Truls,Sendersky Veronica,Hutchison Steve,Allen LaPointe Nancy M,Al-Khatib Sana M,Usdin Yasuda Sally,Dans Peter E,Wright Alan,Califf Robert M,Woosley Raymond L,Schulman Kevin A]Am J Med,2003 Feb 1;114(2):135-41. PMID: 12586234
27: Divergent proarrhythmic potential of macrolide antibiotics despite similar QT prolongation: fast phase 3 repolarization prevents early afterdepolarizations and torsade de pointes.
[Milberg Peter,Eckardt Lars,Bruns Hans-Jürgen,Biertz Julia,Ramtin Shahram,Reinsch Nico,Fleischer Dirk,Kirchhof Paulus,Fabritz Larissa,Breithardt Günter,Haverkamp Wilhelm]J Pharmacol Exp Ther,2002 Oct;303(1):218-25. PMID: 12235254
28: [Ventricular tachycardia and long QT associated with clarithromycin administration in a patient with HIV infection].
[Vallejo Camazón Nuria,Rodríguez Pardo Dolors,Sánchez Hidalgo Antonio,Tornos Mas María Pilar,Ribera Esteban,Soler Soler Jordi]Rev Esp Cardiol,2002 Aug;55(8):878-81. PMID: 12199987
29: Blockade of human cardiac potassium channel human ether-a-go-go-related gene (HERG) by macrolide antibiotics.
[Volberg Walter A,Koci Bryan J,Su Weiguo,Lin Jing,Zhou Jun]J Pharmacol Exp Ther,2002 Jul;302(1):320-7. PMID: 12065733
30: Clarithromycin associated with torsades de pointes.
[Kamochi H,Nii T,Eguchi K,Mori T,Yamamoto A,Shimoda K,Ibaraki K]Jpn Circ J,1999 May;63(5):421-2. PMID: 10943628
31: Drug interactions with cisapride: clinical implications.
[Michalets E L,Williams C R]Clin Pharmacokinet,2000 Jul;39(1):49-75. PMID: 10926350
32: Moxifloxacin: a review of its clinical potential in the management of community-acquired respiratory tract infections.
[Balfour J A,Lamb H M]Drugs,2000 Jan;59(1):115-39. PMID: 10718103
33: Pharmacokinetic-pharmacodynamic consequences and clinical relevance of cytochrome P450 3A4 inhibition.
[Dresser G K,Spence J D,Bailey D G]Clin Pharmacokinet,2000 Jan;38(1):41-57. PMID: 10668858
34: Torsade de pointes induced by cisapride/clarithromycin interaction.
[Piquette R K]Ann Pharmacother,1999 Jan;33(1):22-6. PMID: 9972380
35: The influence of cisapride and clarithromycin on QT intervals in healthy volunteers.
[van Haarst A D,van 't Klooster G A,van Gerven J M,Schoemaker R C,van Oene J C,Burggraaf J,Coene M C,Cohen A F]Clin Pharmacol Ther,1998 Nov;64(5):542-6. PMID: 9834046
36: Syncopal episodes associated with cisapride and concurrent drugs.
[Gray V S]Ann Pharmacother,1998 Jun;32(6):648-51. PMID: 9640482
37: QT prolongation and Torsades de Pointes associated with clarithromycin.
[Lee K L,Jim M H,Tang S C,Tai Y T]Am J Med,1998 Apr;104(4):395-6. PMID: 9576415
Disclaimer:
The content of this database of QT prolongation is intended for educational and scientific research purposes only. It is not intended as a substitute for professional medical advice, diagnosis or treatment.
Any mention of commercial products is for clarification and not intended as endorsement.