Search for drugs:
Typing the drug name to query
VENLAFAXINE
DIR Classification
Classification:Moderate-DIQT concern
Severity Score:2.0
Description in Drug Labeling: View Full Labeling: SPL in DailyMed | PDF
- PRECAUTIONS
- The electrocardiograms for 357 patients who received venlafaxine hydrochloride extended-release capsules and 285 patients who received placebo in 8 to 12 week double-blind, placebo-controlled trials were analyzed. The mean change from baseline in corrected QT interval (QTc) for venlafaxine hydrochloride extended-release capsule-treated patients was increased relative to that for placebo-treated patients (increase of 4.7 msec for venlafaxine hydrochloride extended-release capsules and decrease of 1.9 msec for placebo). In these same trials, the mean change from baseline in heart rate for venlafaxine hydrochloride extended-release capsule-treated patients was significantly higher than that for placebo (a mean increase of 4 beats per minute for venlafaxine hydrochloride extended-release capsules and 1 beat per minute for placebo). In a flexible-dose study, with venlafaxine tablets, USP doses in the range of 200 to 375 mg/day and mean dose greater than 300 mg/day, venlafaxine tablets, USP-treated patients had a mean increase in heart rate of 8.5 beats per minute compared with 1.7 beats per minute in the placebo group.
- OVERDOSAGE
- Human Experience
- There were 14 reports of acute overdose with venlafaxine tablets, USP, either alone or in combination with other drugs and/or alcohol, among the patients included in the premarketing evaluation. The majority of the reports involved ingestions in which the total dose of venlafaxine tablets, USP taken was estimated to be no more than several-fold higher than the usual therapeutic dose. The 3 patients who took the highest doses were estimated to have ingested approximately 6.75 g, 2.75 g, and 2.5 g. The resultant peak plasma levels of venlafaxine for the latter 2 patients were 6.24 and 2.35 mcg/mL, respectively, and the peak plasma levels of O-desmethylvenlafaxine were 3.37 and 1.30 mcg/mL, respectively. Plasma venlafaxine levels were not obtained for the patient who ingested 6.75 g of venlafaxine. All 14 patients recovered without sequelae. Most patients reported no symptoms. Among the remaining patients, somnolence was the most commonly reported symptom. The patient who ingested 2.75 g of venlafaxine was observed to have 2 generalized convulsions and a prolongation of QTc to 500 msec, compared with 405 msec at baseline. Mild sinus tachycardia was reported in 2 of the other patients.
- In postmarketing experience, overdose with venlafaxine has occurred predominantly in combination with alcohol and/or other drugs. The most commonly reported events in overdosage include tachycardia, changes in level of consciousness (ranging from somnolence to coma), mydriasis, seizures, and vomiting. Electrocardiogram changes (e.g., prolongation of QT interval, bundle branch block, QRS prolongation), ventricular tachycardia, bradycardia, hypotension, rhabdomyolysis, vertigo, liver necrosis, serotonin syndrome, and death have been reported.
- ADVERSE REACTIONS
- Postmarketing Reports
- Voluntary reports of other adverse events temporally associated with the use of venlafaxine that have been received since market introduction and that may have no causal relationship with the use of venlafaxine include the following: agranulocytosis, anaphylaxis, angioedema, aplastic anemia, catatonia, congenital anomalies, impaired coordination and balance, CPK increased, deep vein thrombophlebitis, delirium, EKG abnormalities such as QT prolongation; cardiac arrhythmias including atrial fibrillation, supraventricular tachycardia, ventricular extrasystole, and rare reports of ventricular fibrillation and ventricular tachycardia, including torsade de pointes; toxic epidermal necrolysis/Stevens-Johnson syndrome, erythema multiforme, extrapyramidal symptoms (including dyskinesia and tardive dyskinesia), angle-closure glaucoma, hemorrhage (including eye and gastrointestinal bleeding), hepatic events (including GGT elevation; abnormalities of unspecified liver function tests; liver damage, necrosis, or failure; and fatty liver), interstitial lung disease, involuntary movements, LDH increased, neutropenia, night sweats, pancreatitis, pancytopenia, panic, prolactin increased, renal failure, rhabdomyolysis, shock-like electrical sensations or tinnitus (in some cases, subsequent to the discontinuation of venlafaxine or tapering of dose), and syndrome of inappropriate antidiuretic hormone secretion (usually in the elderly).
Postmarketing Surveillance
Contingency Table:
Current Drug
Other Drugs
QT Prolongation
284
23808
Other ADRs
88621
38292966
Odds Ratio = 5.155
Drug Property Information
ATC Code(s):
- N06AX16 - venlafaxine
- N06AX - Other antidepressants
- N06A - ANTIDEPRESSANTS
- N06 - PSYCHOANALEPTICS
- N - NERVOUS SYSTEM
Active Ingredient:VENLAFAXINE HYDROCHLORIDE
Active Ingredient UNII:7D7RX5A8MO
Drugbank ID:DB00285
PubChem Compound:5656
CTD ID:D000069470
PharmGKB:PA451866
CAS Number:93413-69-5
Dosage Form(s):tablet
Route(s) Of Administrator:oral
Daily Dose:
- 100.0 mg/day N06AX16
Chemical Structure: 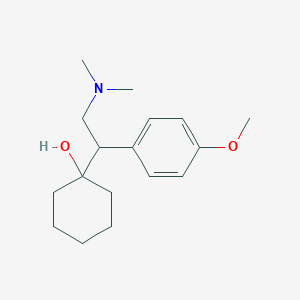

SMILE Code:
COC1=CC=C(C=C1)C(CN(C)C)C1(O)CCCCC1
COC1=CC=C(C=C1)C(CN(C)C)C1(O)CCCCC1
Reference
1: Arrhythmias related to antipsychotics and antidepressants: an analysis of the summaries of product characteristics of original products approved in Germany.
[Elsayed Mohamed,Abdel-Kahaar Emaad,Gahr Maximilian,Connemann Bernhard J,Denkinger Michael,Schönfeldt-Lecuona Carlos]Eur J Clin Pharmacol,2021 May;77(5):767-775. PMID: 33230596
2: Effects of antidepressants on QT interval in people with mental disorders.
[Aronow Wilbert S,Shamliyan Tatyana A]Arch Med Sci,2020 May 29;16(4):727-741. PMID: 32542073
3: QT prolongation in patients with acute leukemia or high-risk myelodysplastic syndrome prescribed antifungal prophylaxis during chemotherapy-induced neutropenia.
[Barreto Jason N,Cullen Michael W,Mara Kristin C,Grove Meagan E,Sierzchulski Amanda G,Dahl Nathan J,Tosh Pritish K,Dierkhising Ross A,Patnaik Mrinal M,Ackerman Michael J]Leuk Lymphoma,2019 Dec;60(14):3512-3520. PMID: 31298598
4: Distribution of Eight QT-Prolonging Drugs and Their Main Metabolites Between Postmortem Cardiac Tissue and Blood Reveals Potential Pitfalls in Toxicological Interpretation.
[Mikkelsen Christian R,Jornil Jakob R,Andersen Ljubica V,Banner Jytte,Hasselstrøm Jørgen B]J Anal Toxicol,2018 Jul 1;42(6):375-383. PMID: 29579279
5: QTc Time Correlates with Amitriptyline and Venlafaxine Serum Levels in Elderly Psychiatric Inpatients.
[Hefner Gudrun,Hahn Martina,Hohner Matthias,Roll Sybille C,Klimke Ansgar,Hiemke Christoph]Pharmacopsychiatry,2019 Jan;52(1):38-43. PMID: 29466824
6: Association of QT-Prolonging Medication Use in CKD with Electrocardiographic Manifestations.
[Snitker Soren,Doerfler Rebecca M,Soliman Elsayed Z,Deo Rajat,St Peter Wendy L,Kramlik Susan,Fischer Michael J,Navaneethan Sankar,Delafontaine Patrice,Jaar Bernard G,Ojo Akinlolu,Makos Gail K,Slaven Anne,Weir Matthew R,Zhan Min,Fink Jeffrey C,for CRIC Study Investigators]Clin J Am Soc Nephrol,2017 Sep 7;12(9):1409-1417. PMID: 28793999
7: A Pilot Study: Cardiac Parameters in Children Receiving New-Generation Antidepressants.
[Uchida Mai,Spencer Andrea E,Kenworthy Tara,Chan James,Fitzgerald Maura,Rosales Ana Maria,Kagan Elana,Saunders Alexandra,Biederman Joseph]J Clin Psychopharmacol,2017 Jun;37(3):359-362. PMID: 28301398
8: Venlafaxine and takotsubo syndrome: Can we learn more from published patient cases?
[Madias John E]Int J Cardiol,2016 Dec 15;225:73-74. PMID: 27716552
9: Drugs with potential cardiac adverse effects: Retrospective study in a large cohort of parkinsonian patients.
[Heranval A,Lefaucheur R,Fetter D,Rouillé A,Le Goff F,Maltête D]Rev Neurol (Paris),Apr-May 2016;172(4-5):318-23. PMID: 27063094
10: Venlafaxine induced QTc interval prolongation in a therapeutic dose.
[Bavle Amar]Asian J Psychiatr,2015 Aug;16:63-4. PMID: 26187237
11: Levomilnacipran for the treatment of major depressive disorder: a review.
[Asnis Gregory M,Henderson Margaret A]Neuropsychiatr Dis Treat,2015 Jan 9;11:125-35. PMID: 25657584
12: Risk of QT/QTc prolongation among newer non-SSRI antidepressants.
[Jasiak Natalia M,Bostwick Jolene R]Ann Pharmacother,2014 Dec;48(12):1620-8. PMID: 25204465
13: QTc prolongation by psychotropic drugs and the risk of Torsade de Pointes.
[Wenzel-Seifert Katharina,Wittmann Markus,Haen Ekkehard]Dtsch Arztebl Int,2011 Oct;108(41):687-93. PMID: 22114630
14: Digital Holter measurement of QT prolongation in ziprasidone overdose.
[Berling Ingrid,Isbister Geoffrey K,Calver Leonie,Clunas Sally]Clin Toxicol (Phila),2011 Aug;49(7):694-6. PMID: 21819290
15: QT interval prolongation associated with venlafaxine administration.
[Letsas Konstantinos,Korantzopoulos Panagiotis,Pappas Loucas,Evangelou Dimitrios,Efremidis Michalis,Kardaras Fotis]Int J Cardiol,2006 Apr 28;109(1):116-7. PMID: 16574528
16: Antidepressants: their effects on cardiac channels, QT prolongation and Torsade de Pointes.
[Sala Michela,Coppa Fabio,Cappucciati Corrado,Brambilla Paolo,d'Allio Giorgio,Caverzasi Edgardo,Barale Francesco,De Ferrari Gaetano M]Curr Opin Investig Drugs,2006 Mar;7(3):256-63. PMID: 16555686
17: QT interval prolongation related to psychoactive drug treatment: a comparison of monotherapy versus polytherapy.
[Sala Michela,Vicentini Alessandro,Brambilla Paolo,Montomoli Cristina,Jogia Jigar Rs,Caverzasi Eduardo,Bonzano Alberto,Piccinelli Marco,Barale Francesco,De Ferrari Gaetano M]Ann Gen Psychiatry,2005 Jan 25;4(1):1. PMID: 15845138
18: Comparative toxicity of citalopram and the newer antidepressants after overdose.
[Kelly C A,Dhaun N,Laing W J,Strachan F E,Good A M,Bateman D N]J Toxicol Clin Toxicol,2004;42(1):67-71. PMID: 15083939
19: Asymptomatic QTc prolongation associated with quetiapine fumarate overdose in a patient being treated with risperidone.
[Beelen A P,Yeo K T,Lewis L D]Hum Exp Toxicol,2001 Apr;20(4):215-9. PMID: 11393275
Disclaimer:
The content of this database of QT prolongation is intended for educational and scientific research purposes only. It is not intended as a substitute for professional medical advice, diagnosis or treatment.
Any mention of commercial products is for clarification and not intended as endorsement.