Search for drugs:
Typing the drug name to query
PITOLISANT HYDROCHLORIDE
DIR Classification
Classification:Moderate-DIQT concern
Severity Score:3.0
Description in Drug Labeling: View Full Labeling: SPL in DailyMed | PDF
- WARNINGS AND PRECAUTIONS
- QT Interval Prolongation
- WAKIX prolongs the QT interval. The use of WAKIX should be avoided in patients with known QT prolongation or in combination with other drugs known to prolong the QT interval [see Drug Interactions (7.1)]. WAKIX should also be avoided in patients with a history of cardiac arrhythmias, as well as other circumstances that may increase the risk of the occurrence of torsade de pointes or sudden death, including symptomatic bradycardia, hypokalemia or hypomagnesemia, and the presence of congenital prolongation of the QT interval [see Clinical Pharmacology (12.2)]. The risk of QT prolongation may be greater in patients with hepatic or renal impairment due to higher concentrations of pitolisant. Monitor patients with hepatic or renal impairment for increased QTc. Dosage modification is recommended in patients with moderate hepatic impairment and moderate or severe renal impairment [see Dosage and Administration (2.2, 2.3)]. WAKIX is contraindicated in patients with severe hepatic impairment [see Contraindications (4)]. WAKIX is not recommended in patients with end-stage renal disease (ESRD) [see Dosage and Administration (2.3), Use in Specific Populations (8.7), Clinical Pharmacology (12.3)].
- DRUG INTERACTIONS
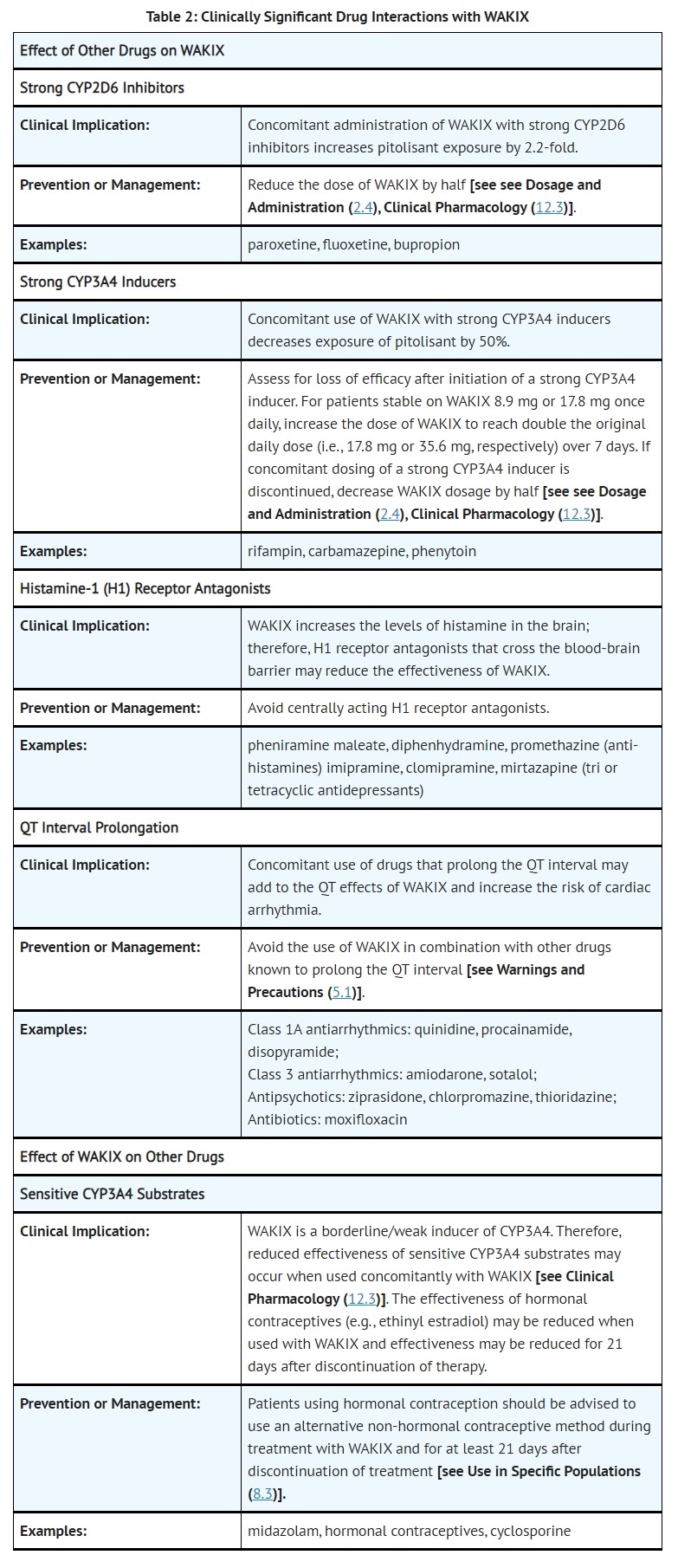
- ADVERSE REACTIONS
- The following adverse reactions are discussed in more detail in other sections of the labeling:
- QT Interval Prolongation [see Warnings and Precautions (5.1)]
- CLINICAL PHARMACOLOGY
- Pharmacodynamics
- Cardiac Electrophysiology
- WAKIX at the highest recommended dosage (i.e., 35.6 mg daily) led to a QTc increase of 4.2 msec. Exposures 3.8-fold higher than achieved at the highest recommended dose increase QTc 16 msec (mean) [see Warnings and Precautions (5.1)].
- PATIENT COUNSELING INFORMATION
- Prolongation of the QT Interval
- Inform patients to consult their physician immediately if they feel faint, lose consciousness, or have heart palpitations [see Warnings and Precautions (5.1)]. Advise patients to inform their healthcare provider that they are taking WAKIX before any new drug is taken.
Postmarketing Surveillance
Contingency Table:
Current Drug
Other Drugs
QT Prolongation
1
24091
Other ADRs
1224
38380363
Odds Ratio = 1.302
Drug Property Information
ATC Code(s):
- N07XX11 - pitolisant hydrochloride
- N07XX - Other nervous system drugs
- N07X - OTHER NERVOUS SYSTEM DRUGS
- N07 - OTHER NERVOUS SYSTEM DRUGS
- N - NERVOUS SYSTEM
Active Ingredient:PITOLISANT HYDROCHLORIDE
Active Ingredient UNII:YV33CH63HI
Drugbank ID:DB11642
PubChem Compound:N/ADIR Classification
CTD ID:C516975
CAS Number:362665-56-3
Dosage Form(s):tablet, film coated
Route(s) Of Administrator:oral
Daily Dose:
- 18.0 mg/day N07XX11
Chemical Structure: 

SMILE Code:
ClC1=CC=C(CCCOCCCN2CCCCC2)C=C1
ClC1=CC=C(CCCOCCCN2CCCCC2)C=C1
Reference
N/A
Disclaimer:
The content of this database of QT prolongation is intended for educational and scientific research purposes only. It is not intended as a substitute for professional medical advice, diagnosis or treatment.
Any mention of commercial products is for clarification and not intended as endorsement.